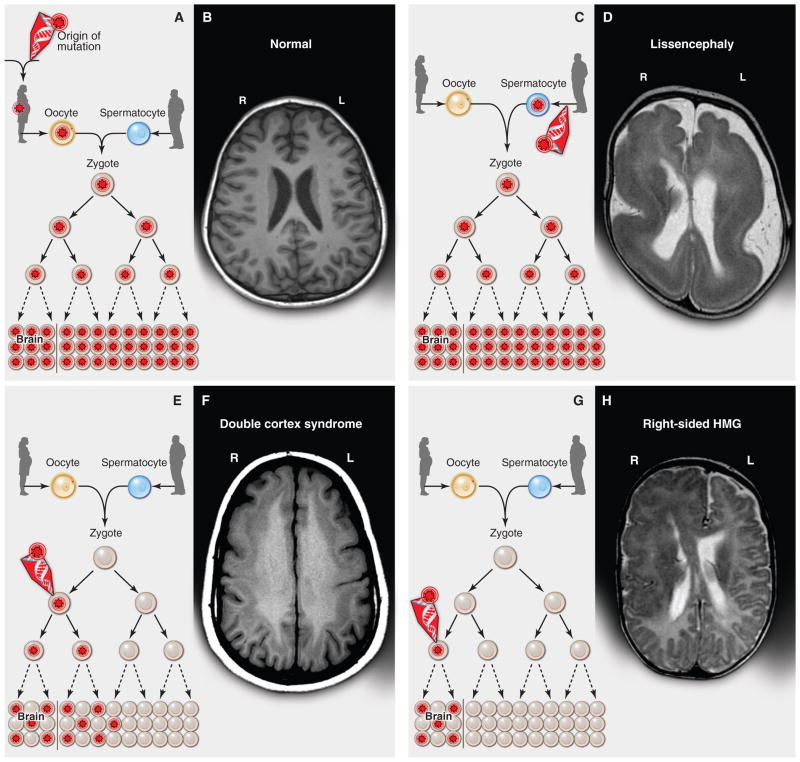
Fig. 1. Inherited, de novo, and somatic mutations causing neurological disease.
(A) A heterozygous mutation is inherited from one parent. This mechanism is typical of autosomal dominant epilepsy. In this example, the mutation originally presented in the mother, whose oocytes in turn carry the mutation. (The mutation arose during gametogenesis in one of the parents of the mother, top left.) It is present in the zygote and thus all cells of the affected child. (B) This axial T1-weighted image from a MRI study of a patient with inherited epilepsy appears normal. Individuals with dominantly inherited epilepsies caused by mutations in genes encoding ion channels, for example, have normal neuroimaging studies despite every cell carrying a mutation. (C) A de novo mutation may arise sporadically during gametogenesis, in this case spermatogenesis. This mechanism of mutation would be typical of a de novo mutation in the gene SCN1A associated with severe myoclonic epilepsy of infancy or LIS1 associated with lissencephaly. Even though every cell in the individual carries the mutation, the predominant effects of the mutation depend on the distribution of gene expression; in these examples, the brain is primarily affected. (D) An axial T2-weighted MRI image shows the severe gyral simplification—more pronounced posteriorly (the bottom of the figure)—that is associated with mutations in the gene LIS1. (E) An early post-zygotic mutation results in a mutation present in most or all tissues of the organism (including the leukocytes, which are generally assayed for clinical genetic testing) but in a mosaic fashion, with only a portion of all cells in each tissue harboring the mutation. This pattern, illustrated by the axial T1-weighted image in (F), has been observed in mosaic cases of double cortex syndrome involving the gene DCX. Visible is the extra band of gray matter underlying the normal-appearing outer aspect of the cerebral cortex. Because DCX is required for normal migration of neurons from the ventricular region deep in the brain to the superficial cortex, the cells carrying the DCX mutation only migrate about halfway to the cortex and then arrest their migration. (G) A late post-zygotic mutation will be present in only certain tissues in a mosaic fashion, in this case apparently in half of the brain. This is the pattern observed in some cases of HMG with somatic mosaic point mutations in AKT3 and other related genes and somatic mosaic increase of copy number of chromosome 1q. (H) This axial T2-weighted MRI image shows right-sided HMG, characterized here by enlargement of the right hemisphere, abnormally thick and dark-appearing gray matter anteriorly, heterotopic periventricular gray matter, and abnormal white matter signal in the right hemisphere. (R, right; L, left).