Figure 1.
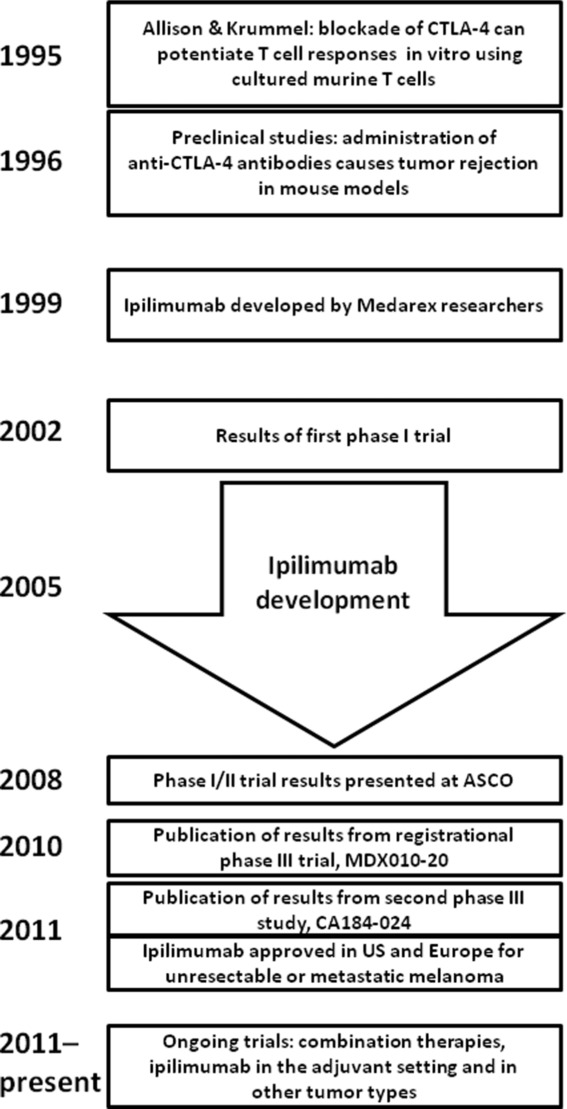
Key milestones in the development of ipilimumab. Following preclinical studies of CTLA-4 blockade in murine tumor models, ipilimumab was developed as a fully human monoclonal antibody and evaluated in several clinical trials. These trials included two randomized, controlled phase III trials in which ipilimumab demonstrated an improvement in OS. Ipilimumab received approval in 2011 in both the United States and the European Union for the treatment of patients with unresectable or metastatic melanoma. Ongoing evaluation of ipilimumab includes combination studies with other anticancer therapies (e.g., RT, anti-PD-1 antibody), as an adjuvant therapy for stage III melanoma, and its efficacy and safety in several other solid tumors.
