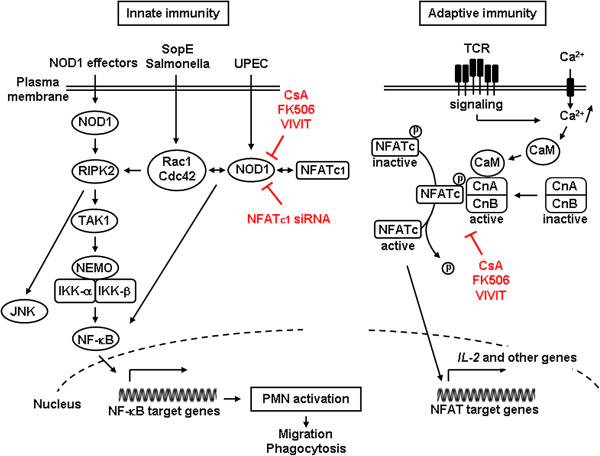
Figure 2.
NOD1 signaling and interactions with Rho GTPases and NFATc. (Left panel) Schematic representation of the activation of NOD1 interacting with Caspase recruitment domain (CARD)-containing kinase RIPK2, which leads to activation of RIPK2 and subsequent recruitment and activation of TAK1. The TAK1 complex then induces the polyubiquitination of the IKK-β kinase, degradation of the NF-κB repressor IκB, nuclear translocation of NF-Kb, and transcription of pro-inflammatory mediators. NOD1 stimulating agonists also activate JNK. The guanine exchanger SopE from salmonella activates Rho GTPase Rac1 and Cdc42, which form a protein complex with NOD1 and the heat shock protein 90 (not shown). Activation of Rac1 and Cdc42 induces the activation of NOD1/RIPK2 signaling and induction of NF-κB-mediated pro-inflammatory mediators. Downregulation of NOD1 expression by calcineurin inhibitors or silencing NFATc1 mRNA markedly impairs bacterial-mediated activation of PMN functions (e.g. neutrophil migration and E. coli phagocytic killing capacities). (Right panel) Schematic view of the NFATc activation pathway by cell surface receptors coupled to Ca2+ mobilization. Ca2+-dependent activation of calmodulin (CaM) and calcineurin B (CnB) enables CaM binding to CnA regulatory region, and CnA activating conformational change. Activated calcineurin then dephosphorylates the phosphorylation motifs of NFATc, allowing NFATc to translocate to the nucleus. Nuclear NFATc, in collaboration with other transcription factors (such as AP-1), then induce gene transcription. Calcineurin activity is inhibited by CsA or FK506, which binds to their intracellular immunophillin receptors. The high-affinity calcineurin-binding VIVIT peptides are potent blockers of NFATc. IKK: I kappa B kinase; JNK: c-Jun N-terminal kinases; NEMO: NF-κB essential modulator; NF-κB: nuclear factor κB; RIPK2: Receptor-interacting serine/threonine-protein kinase 2; TAK1: Transforming growth factor-beta-activated kinase 1; TCR: T cell receptor.