Abstract
Background & Aims
US guidelines recommend surveillance of patients with Barrett's esophagus (BE) to detect dysplasia. BE is conventionally monitored via white-light endoscopy (WLE) and collection of random biopsies. However, this approach does not definitively or consistently detect areas of dysplasia. Advanced imaging technologies can increase detection of dysplasia and cancer. We investigated whether these can increase the diagnostic yield for detection of neoplasia in patients with BE, compared with WLE and analysis of random biopsies.
Methods
We performed a systematic review, using Medline and Embase to identify relevant peer-review studies. Fourteen studies were included in the final analysis, with a total of 843 patients. Our metameter (estimate) of interest was the paired-risk difference (RD), defined as the difference in yield of detection of dysplasia or cancer using advanced imaging vs WLE. The estimated paired-RD and 95% confidence interval (CI) were obtained using random effects models. Heterogeneity was assessed by means of the Q statistic and I2 statistic. An exploratory meta-regression was performed to look for associations between the metameter and potential confounders or modifiers.
Results
Overall, advanced imaging techniques increased the diagnostic yield for detection of dysplasia or cancer by 34% (95% CI, 20%–56%; P<.0001). A subgroup analysis showed that virtual chromoendoscopy significantly increased diagnostic yield (RD=0.34; 95% CI, 0.14 – 0.56; P<.0001). The RD for chromoendoscopy was 0.35 (0.13–0.56; P=.0001). There was no significant difference between virtual chromoendoscopy and chromoendoscopy, based on Student t test analysis (P=.45).
Conclusions
Based on a meta-analysis, advanced imaging techniques such as chromoendoscopy or virtual chromoendoscopy significantly increase diagnostic yield for identification of dysplasia or cancer in patients with BE.
Keywords: Barrett's esophagus, PRISMA, QUADAS, advanced imaging, risk difference, esophageal adenocarcinoma
Introduction
Barrett esophagus (BE), also known as intestinal metaplasia of the tubular esophagus, is a major risk factor for the development of esophageal adenocarcinoma (EAC).1-3 The incidence of EAC has been steadily increasing.4-7 The evolution of BE to the EAC progresses through a sequence of low-grade dysplasia (LGD) to high grade dysplasia (HGD) and eventually adenocarcinoma.8 Surveillance is recommended in patients with history of BE.9-11 Current surveillance practice standards require random, four-quadrant biopsy every 1-2 cm of BE (Seattle Protocol) to look for dysplasia with assistance of white light endoscopy (WLE).9, 12 However, this approach is both labor intensive and of low yield.13 Therefore, advanced imaging modalities have been studied in an attempt to improve detection of BE dysplasia. The most studied techniques are: chromoendoscopy, virtual chromoendoscopy, and confocal laser endomicroscopy.
Chromoendoscopy (CE) utilizes dyes to improve visualization of the esophageal mucosa. The dyes enhance the mucosal patterns associated with dysplasia, thus enabling better detection during endoscopy. Dyes presently used in practice include: indigo carmine, methylene blue, crystal violet, and acetic acid.14 On the other hand, instead of using dye, virtual chromoendoscopy (VC), employs the use of light filters within the endoscope to highlight vessel and mucosa patterns. VC includes: narrow band imaging (NBI, by Olympus)15, and Fujinon intelligent chromoendoscopy (FICE, by Fujinon®).16 Confocal laser endomicroscopy (CLE) magnifies the gross image of the esophageal mucosa by a thousand-fold. This magnification allows the endoscopist to visualize the mucosa at the microscopic, cellular level. With CLE, the image is seen as a real-time optical microscopy. CLE can be probe-based (through the working channel of an endoscope) or incorporated into the endoscope.14
Our objective was to carry out a systematic review and meta-analysis on existing studies, evaluating the yield of advanced surface imaging modalities (CE and VC) in the detection of esophageal dysplasia/neoplasia compared to the current standard of care (WLE/RB). Because of the inherit differences between CE/VC and CLE, studies of CLE were not included in this analysis. We hypothesize that use of any of the newer imaging modalities will increase the diagnostic yield compared to random biopsies.
Methods
Study Selection
In conducting this study, we followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines17 and the guidance provided by Kanwal et. al.18 We included all studies that comparatively evaluated both WLE/RB and either one of the new imaging modality (i.e. CE or VC) for the detection of dysplastic changes in patients with BE. Our inclusion criteria included: (i) prospective clinical studies and randomized controlled trials; (ii) studies that were published in peer-reviewed journals; (iii) studies that had the assessment of dysplasia and/or non-invasive EAC as one of their outcomes; (iv) studies that included both WLE with random biopsy and CE (or VC) with targeted biopsies; and (v) studies with extractable information regarding the diagnostic yield of WLE vs. CE (or VC).
Studies were excluded if: (i) no random biopsies were performed or if the diagnostic yield was not extractable from the study design; (ii) diagnostic yield assessment was done on a per-lesion basis with no results on a per-patient basis. (iii) if the outcome reported was intestinal metaplasia, and not dysplasia or neoplasia.
Search Strategy and Data Extraction
The search was done by four independent reviewers (BQ, RU, HW, NB). Medline and Embase databases were searched for articles online. The search was limited to human subjects and the English language. Search terms are included in Appendix (Appendix 1). The last date of search was 10/1/2012. References from both databases were imported into EndNote (Thomson Reuters, Carlsbad, CA). Duplicates were reviewed and removed. Studies were reviewed by title and abstract. Studies were excluded if they were not original articles (i.e. reviews, case reports, case series, editorials, abstracts, or conference papers), or were irrelevant to the study topic (Figure 1). Thirty-four articles were reviewed in full text for the CE group, and fifty-three in the VC group. Based on review of the full text, seven papers were included in the final meta-analysis from the CE group and seven from the VC group for a total of 14 studies.
Figure 1.
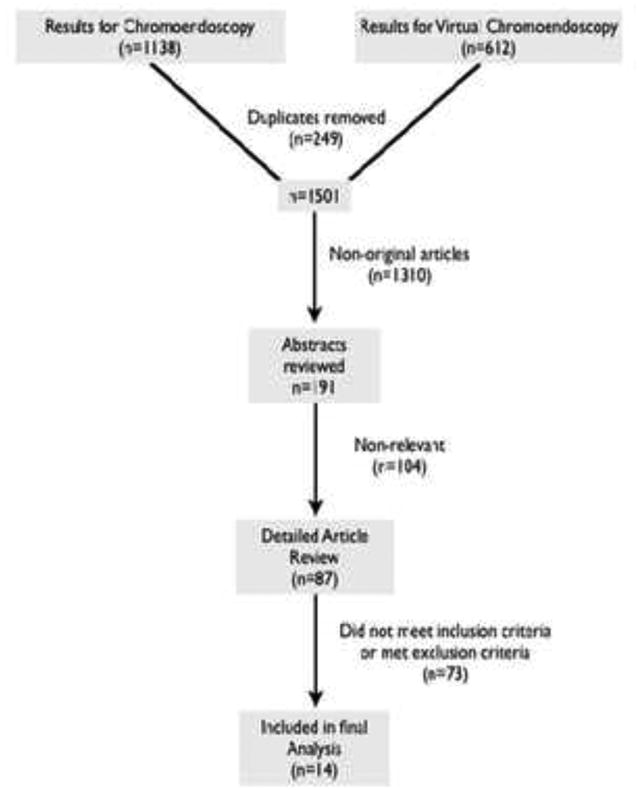
Flow diagram demonstrating assessment and selection of studies in the meta-analysis.
For each study the following data was extracted: primary author, publication journal and year, country/countries where the study was done, study design, endoscopic equipment, advanced imaging modality, type of dye used (if applicable), prevalence of dysplasia in the study population, distribution of patient age, patient gender, race (if reported), number of patients who were analyzed for dysplasia by CE/VC and random biopsy, number of patients found to have dysplasia using advanced imaging vs. random biopsy, number of endoscopists in the study, number of pathologists and whether they were blinded to the biopsy method, and the total number of biopsies taken in each group (or mean/median if totals are not reported).
Quality Assessment
To assess the quality of each study, the Quality Assessment of Diagnostic Accuracy Studies (QUADAS)19 was used. Each study was assessed by two independent reviewers and the scores were then averaged. The responses to the answers were either “yes,” “no,” or “unclear.” A study was given a point for every “yes” response, half a point for “unclear,” and no points for “no;” maximum points awarded to a study=14.
Statistical Analysis
Data from the evidence table was used to perform the meta-analysis using CMA software (Biostat Inc, Englewood, NJ). The risk difference in the diagnostic yield for dysplasia detection when using AI compared to current standard of care was used as the primary outcome of interest. This outcome is clinically relevant and is not biased by the lack of true dysplasia status. Test characteristics like sensitivity and specificity are biased by this lack for true disease status (some patients with dysplasia are missed by random and targeted biopsies). We defined the risk difference as the proportion of patients with dysplasia on advanced imaging minus the proportion of patients with dysplasia on white light with random biopsies. Since most studies were crossover, matched proportions were used for our analysis. To do such analysis, the external correlation needed to be known. Yet, none of the studies reported such figure. Therefore, an external correlation of 0.5 (halfway between no correlation and complete correlation) was used in the analysis. A sensitivity analysis was done by varying the correlation coefficient and observing the change in the effect size of the metameter. The estimated size effect of the metameter was reported using fixed and random effects models with 95% CI. Forest plots were formulated to contrast effect sizes in each of the studies. Q statistic and I2 tests were calculated to assess heterogeneity between studies. Random effect models were used when test of heterogeneity was significant (I2 >50% or p <0.1 for Q statistic). Fixed effect models were otherwise used. A subgroup analysis was performed for the VC and CE groups and t-test was used to assess differences between the two groups. A funnel plot with the trim-and-fill method was used to screen for possible small study or publication bias. The classic fail-safe test was used to assess the number unpublished studies needed to negate the observed effects. An exploratory univariate meta-regression was done to assess the association between the ratio of total biopsies in AI and RB, gender, race, and number of endoscopists.
Several factors were considered a priori as possible sources of heterogeneity:
Imaging modality: clearly VC is different from CE. Therefore, a subgroup analysis was planned to assess the difference between the two modalities using ANOVA.
Type of dye used: some of the heterogeneity could be due to the different dyes used in the different studies (methylene blue vs. acetic acid vs. indigo carmine, etc.)
Different image enhancement technologies in VC group (NBI, FICE, etc.).
Study population: studies were done in different countries, with prevalence of dysplasia potentially differing across these populations.
Number of biopsies: number of biopsies performed could be linked to the diagnostic yield of random biopsies and targeted biopsies.
Experience of endoscopist: endoscopists with more experience in recognizing the abnormalities on AI may show better outcomes compared to those with less experience.
Results
Study Selection
The process of study selection is summarized in figure 1. In total, we reviewed the full text for 87 manuscripts. Among those, only 15 studies met the inclusion criteria; however, an additional study20 was removed from the final analysis due to the prolonged time (6 months) between the RB and the CE target biopsy. Therefore, a total of 14 studies21-34 were analyzed (7 VC studies, 7 CE). In total, 843 patients were included in the analysis. Patient, procedure, and study characteristics are summarized in Table 1. The quality of each study based on the QUADAS tool is reported in Supplemental Table 1.
Table 1. patient and study characteristics for the 14 studies included in the meta-analysis.
| Author (Year) | Country | Study Design | Technology | Equipment | # Patients | % Male | % White | Age | Population | # Endoscopists | # Blinded pathologist | Dysp. AI | Total AI | Dysp. WLE | Total WLE | Biopsies AI | Biopsies WLE |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Canto, M. (2000) | USA | RCT (crossover) | MB | 1T-100 or 2T-100 (Olympus) | 43 | 74% | 95% | 60 (31 -70) | BE | - | Yes (2) | 19 | 43 | 12 | 43 | 563 | 385 |
| Grossner L. (2006) | Germany | RCT (cross-over) | MB | 450 HR (Fujinon) | 86 | 87% | - | 65 ± 8 | BE with HGD or AC | - | No (2) | 75 | 86 | 56 | 86 | 562 | 1217 |
| Horwhat, J. (2007) | USA | RCT (crossover) | MB | GIF 130 or 140, GIFQ 140: (Olympus) | 48 | 92% | 96% | 62 ± 11 | BE with HGD or AC | 48 | Yes (1) | 16 | 48 | 18 | 48 | 439 | 917 |
| Kara, M. (2005) | Nether-lands | RCT (crossover) | IC | GIF-Q240Z (Olympus) | 28 | 86% | - | 66 ±10 | BE ± dysp./neoplasia | 4 | Yes (2) | 11 | 14 | 9 | 14 | - | - |
| Ragunath, K. (2003) | UK | RCT (crossover) | MB | Olympus or Fujinon | 57 | 77% | - | 60 (31-85) | GERD or BE | 2 | Yes (2) | 26 | 57 | 23 | 57 | 651 | 618 |
| Fortun P. (2006) | UK | Post hoc analysis of images | AA | GIF Q240Z (Olympus) | 64 | 59% | - | 62 (26-83) | BE | 6 | Yes (1) | 8 | 62 | 2 | 62 | 368 | 357 |
| Wo, J. (2001) | USA | RCT (crossover) | MB | - | 47 | 89% | 93% | 57 ± 11 | GERD or BE | 3 | No (3) | 13 | 35 | 8 | 35 | 287 | 267 |
| Niepsuj, K. (2003) | Poland | Cross-sectional | AFI/LIFE | GIF-E; (Olympus) | 34 | 82% | 95% | 63 (40-77) | BE | 1 | - | 23 | 34 | 18 | 34 | 109 | 136 |
| Camus, M. (2009) | France | Prospective study (retrospective videos) | FICE/AA | EG 490 ZW5 (Fujinon) | 20 | 95% | 96% | 65 ± 15 | BE | 1 | No (3) | 7 | 18 | 1 | 18 | - | - |
| Curvers, W. (2010) | Nether-lands, US, UK | RCT (crossover) | ETMI | XGIF-Q240/GIF-FQ260FZ; (Olympus) | 87 | 82% | - | 67.1 ± 9.1 | BE with HGD/AC | 10 | Yes (5) | 81 | 87 | 69 | 87 | 11.5 | 14 |
| Curvers, W. (2008) | Nether-lands, US, UK | RCT (cross-sectional) | ETMI | XGIF-Q240FZ, (Olympus) | 84 | 83% | - | 67 ± 12 | BE ± Dysplasia | 5 | Yes (≥4) | 27 | 84 | 16 | 84 | - | - |
| Curvers, W. (2011) | Nether-lands | RCT (crossover) | AFI | XGIF-Q240/GIF-FQ260FZ; (Olympus) | 99 | 80% | - | 63 ± 10 | BE ± Dysplasia | 9 | Yes (9) | 60 | 99 | 54 | 99 | - | - |
| Wolfsen, H. (2008) | USA | RCT (tandem) | NBI | GIFH180; GIFQ160 (Olympus) | 65 | 82% | 93% | 66 | BE | 8 | - | 37 | 65 | 28 | 65 | Mean 4.7 | Mean 8.5 |
| Sharma, P. (2012) | US, Netherlands | RCT (crossover) | NBI | GIF-H180 (Olympus) | 123 | 93% | 97% | 63 (38 -85) | BE | - | Yes (1) | 40 | 123 | 35 | 123 | 442 | 934 |
Increase in Diagnostic Yield
Cochran's Q statistic for the whole analysis was 31, p=0.004. I2 was found to be 58%. Both results indicate the presence of heterogeneity between the studies necessitating the use of a random effects analysis. The overall risk difference based on random effects model was 0.34 [0.20 – 0.48], p<0.0001 (Figure 2). Therefore, using advanced imaging, there was a 34% increase in the yield of detecting dysplasia/cancer; consequently, there is a 34% decrease in the risk of missing dysplasia when using advanced imaging. In a subgroup analysis, I2 was 48 for the VC group. On random effects model, VC was found to have a RD of 0.34 [95% CI: 0.14 – 0.54], p=0.001 (Figure 3). This means that VC increases the diagnostic yield of finding dysplasia/cancer by 34% compared to WLE with random biopsies. For the CE group, I2 was 64. Using a random effect model, RD was 0.35 [0.13 – 0.56], p<0.0001 (Figure 3). Therefore, chromoendoscopy appears to increase the diagnostic yield of dysplasia by 35%. Testing for difference in RD between VC and CE failed to detect a significant difference between the two modalities (p=0.57).
Figure 2.
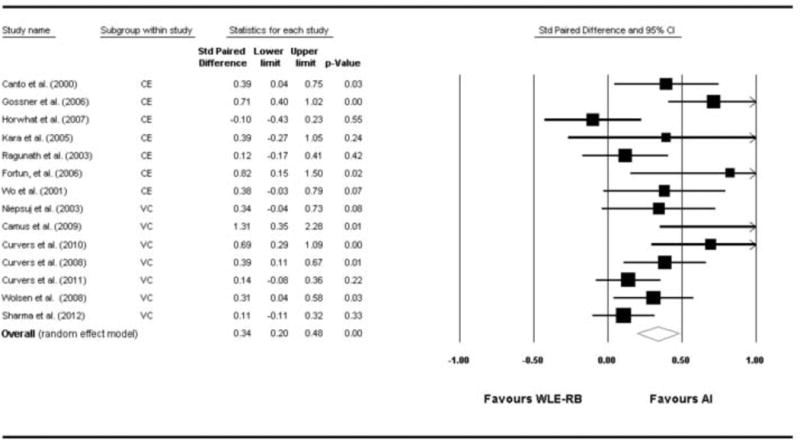
Forest plot of the 14 studies with pooled risk difference for detection of dysplasia by advanced imaging compared to white light with random biopsies.
Figure 3.
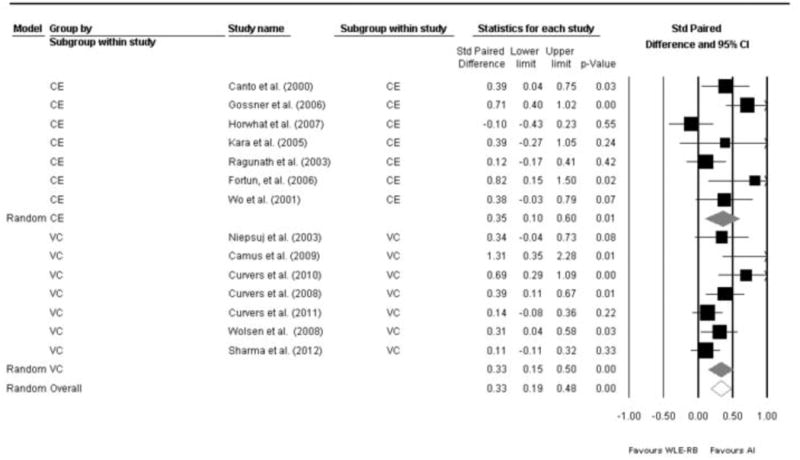
Forest plot of the 14 studies stratified by imaging modality (Virtual Chromoendoscopy in the top 7 studies, compared to chromoendoscopy in the bottom 7 studies) with pooled risk difference. Overall pooled risk difference is also shown.
Small Study or Publication Bias Assessment
A potentially small study or publication bias was assessed using the funnel plot and classic fail-safe test. (Figure 4). Based on graphical assessment, some asymmetry was noted in the funnel plot. The left lower quadrant had less studies and also two studies outside the 95% confidence interval (Grossner et al on the right side and Horwhat et al on the left side of the funnel plot). Therefore, sensitivity analysis was performed to assess how many studies would be needed to negate the significant findings of the analysis. For this purpose, the classic fail-safe test showed that173 “null” studies will be needed in order for the p-value to exceed 0.05. In other words, one would need to find 12 negative studies for each of the identified studies in the meta-analysis in order to negate the significant findings.
Figure 4.
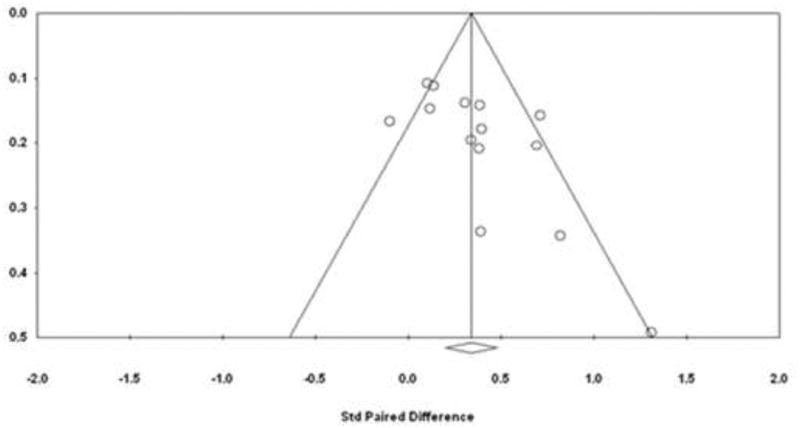
Funnel plot to assess publication bias.
Other Bias
As previously mentioned, a study by Lim et al. was not included in our main analyses because of prolonged follow-up period. However, we included this study in a sub-analysis. The overall effect size was not significantly changed, RD = 0.29 [0.12 − 0.46], p=0.001. Additionally, given that three studies 22, 23, 26 were led by the same author and since some of the patients may have overlapped between those studies (losing the assumption of independence required in meta-analysis), an analysis of influence or leave one out analysis was performed by removing two of the studies at a time (the earliest two studies were removed, then the latest two studies, then the earliest study and the latest study). The RD was found to be similar with minimal deviation (<5%) from the reported effect (data not shown).
The study by Kara et al. compared indigo-carmine CE vs. WLE and NBI vs. WLE. We decide to include the study only once (CE or VC). Including the study in both the CE and the VC groups would violate the assumption of independence between observations. CE and VC each independently picked up dysplasia on 11 of 14 patients. Therefore, although we classified the study as CE, the overall RD would not differ based on how the study was classified.
Sensitivity Analyses
As previously discussed, we used matched binary outcome testing, since most studies were cross-over trials. For that, we used external correlation of 0.5. We tested different external correlation coefficients with resulting RD and CI intervals. Overall, the effect point and CI did not change significantly (<2% in RD, Supplemental Table 2).
In addition we wanted to test the effect of each study on the overall analysis in order to detect and report studies that may have had a large effect on the pooled risk difference. This was done by removing each of the studies and observing the change in the RD. Overall, none of the studies had an overdue influence on the final analysis (Figure 5)
Figure 5.
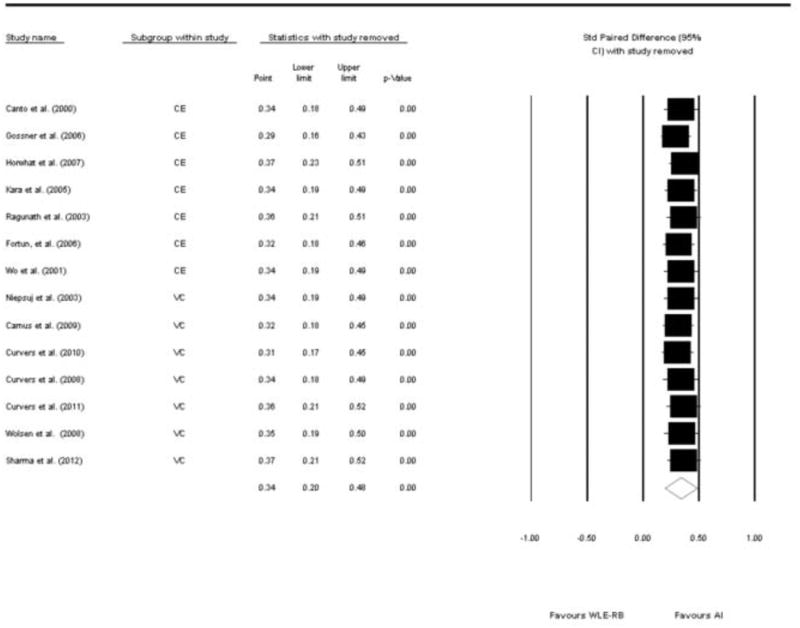
Forest plot of the 14 studies showing the pooled risk difference with one study removed at a time
Meta Regression
We performed a series of univariate meta-regressions to examine the potential relationship between diagnostic yield and each of the following variables: age, gender, the number of endoscopists, QUADAS score, and the ratio between the number of biopsies on AI relative to the number of biopsies on WLE. None were significant independent predictors. However, there was a trend towards increase in diagnostic yield as the ratio of AI biopsies to RB biopsies increased. We also noted that the two studies that showed a much better diagnostic yield in RB compared to CE had very low ratio of AI biopsies to WLE-RB compared to other studies (i.e. the number of biopsies obtained during AI was low compared to the number obtained on WBL-RM). This was also true for the study by Lim et al. where the median number of biopsies for the CE was 4 compared to a median of 11 for WLE-RB. Therefore, we included Lim's study in an expanded exploratory meta regression. This analysis showed a trend towards improved diagnostic yield of AI with more biopsies but (OR 0.3, p=0.049).
Discussion
Clinical Implications
The results of our meta-analysis indicate an increase in the diagnostic yield of dysplasia/cancer (more patients with dysplasia/cancer) when using advanced imaging (VC or CE). In a subgroup analysis, the benefit of VC and CE seemed similar. Overall, heterogeneity was present; therefore, we used random effects models. The effect size of 34% is clinically large enough to warrant considering a change in BE surveillance guidelines.
Among studies identified as possibly being eligible for inclusion in our meta-analysis, a study by Lim et al.20 was excluded from the final analysis for several reasons. First, the time between the two endoscopies was too long (6 months by protocol), which would have introduced confounding into the results. Secondly, the endoscopists took much less time during biopsies during chromoendoscopy than that on random biopsies, much less than all other studies. However, we did include Lim study in a sub-analysis and showed that it did not off-set the benefit of AI despite having a much higher dysplasia yield in the RB group compared to the CE group. The observation of low ratio of AI/WLE biopsies led us to an exploratory meta-regression, which showed a significant correlation between the ratio of AI/WLE biopsies and the yield of dysplasia. This correlation makes sense clinically. The more biopsies obtained, the more likely it is that dysplasia will be detected.
Therefore, we believe that there is evidence to change practice towards AI (chromoendoscopy or virtual chromoendoscopy). Yet, the protocol should include targeted biopsies of any suspicious areas followed by random biopsies based on the Seattle protocol. This may not save time or money on the number of biopsies, but it will have the highest yield for dysplasia/cancer detection. A cost-effectiveness analysis based on such protocol is beyond the scope of this review, but would be an appropriate topic for future studies. The potential to eliminate random biopsy and only perform targeted biopsy has been explored by several investigators and recently reviewed by the American Society for Gastrointestinal Endoscopy. Threshold valued for AI methods were set at least 90% sensitivity and 98% NPV for dysplasia on a per patient basis.35 Although AI systems appear to increase dysplasia detection, most have not shown sufficient accuracy to eliminate concurrent random biopsy.
Our results regarding CE are in agreement with a previous meta-analysis by Ngamreungphong et al 37. They reported an 11% increase in the diagnostic yield for detecting dysplasia (43% in MB vs. 32% in RB). Using the numbers from this study, the calculated paired risk-difference was found to be 26%. This result is lower than our RD of 35%. Such variation is accounted for by the difference in the studies included in each analysis.
There were three studies on the topic of AI with the same lead author. The three studies were done at different time periods, by varying co-investigators, and in different centers. However, these studies may have some overlap in the patient population. Therefore, we did a sub-group analysis in which one of the three studies was kept in the analysis at a time, while the other two were removed. This process was repeated 3 times, thus keeping each one of the three studies in the analysis at any given time. The results of these analyses showed no major difference when compared to the primary analysis (≤ 5% change in RD no change in p-value of <0.0001 regardless of which study was included). Therefore, all three studies were kept in the final analysis.
At low doses used for endoscopy, dyes used for CE appear to be safe. Yet, a study has shown oxidative damage with methylene blue in patients with Barrett's Esophagus.36, 37 This analysis showed that the increased in yield from CE is similar to that of VC. Yet, VC does not have the same concerns of oxidative damage. In addition, VC is easier to perform (e.g., no need to spray and wash dye), and takes only a few seconds to activate by pushing a button on the scope. Therefore, we feel that our results favor the use of VC over CE in surveillance of BE. However,the issue of standardization of VC raises a potential obstacle to wide-spread implementation for this method in screening in patients with BE. As seen in table 1, various imaging modalities were included in the analysis (NBI, AFI, and FICE). Due to the limited number of studies on each of those modalities and the rarity of head-to-head studies among them, we cannot make an assessment of which is better. Based on our results, we conclude that VC is associated with an increased detection of dysplasia/cancer. Similarly, there needs to be a consensus on the proper education of VC to gastroenterologists and trainees in order for VC to be more widely accepted.
Strengths and Limitations
This is the first meta-analysis to consider the increased yield in the diagnosis of dysplasia/cancer among patients with Barrett's Esophagus use CE and VC. Three previous meta-analyses addressed the issue of chromoendoscopy (including VC) and Barrett's esophagus, but looked at VC or CE and not both.37-39 One of those studies dealt with tests characteristics like sensitivity, specificity, and accuracy. While such measures are frequently reported for endoscopic procedures, the true disease status (a ‘gold standard’) for any given patient is typically not known. Dysplasia/cancer can be missed on random biopsies and on chromoendoscopy. Therefore, we do not know for sure that a patient is devoid of dysplasia if he/she has a negative test (i.e., false negatives). Hence, calculating sensitivity, specificity, positive predictive values, and negative predictive value based on those studies is potentially biased. One way to get the true status of disease may be to do complete Barrett excision (CBE) in which the whole Barrett's mucosa is resected endoscopically. CBE has been used as a treatment modality in patients with dysplastic short segment BE (≤3cm) 40. CBE was not done in any of the existing studies. Note that the main concern is missing existing dysplasia/cancer (false negatives). The issue of false positives can also be problematic given the inconsistencies among pathologists in diagnosing dysplasia. This, however, is beyond the scope of this review. We assumed accurate pathological diagnosis in our results and discussion. Given the above limitations, we decided to look at the increased yield in finding dysplasia/cancer in VC/CE when compared to random biopsies. We believe that this measure does not have the same drawbacks as sensitivity and specificity given the absence of a true gold standard; we are merely assessing the difference in cases diagnosed with dysplasia when comparing the two methods. Some studies, however, did not report the yield of CE/VC compared to random biopsies (current standard of care), and were therefore excluded. Most studies were done in older, white males although these are disproportionately affected with BE and thus an appropriate population.
A limitation of our study was the restriction of searches to the English language. We acknowledge this limitation. Reviewing other languages was beyond the scope of this study. Additionally, we did not search conference proceedings and abstracts. Our search strategy included published studies only. Judging the quality of a study based on an abstract is challenging. Therefore, we included published studies only.
Conclusions
Advanced Imaging (Chromoendoscopy or Virtual Chromoendoscopy) appears to offer a significant increase in diagnostic yield with regard to detecting dysplasia/cancer amongst patients with Barrett's Esophagus. Specifically, VC seems to have a more consistent and robust effect. With modern equipment the availability of virtual chromoendoscopy is almost universal. An additional advantage is that VC does not require application of coloring agents, which may be untidy and add extra expense to the procedure.
Based on this meta-analysis, VC may be the test of choice in surveillance of patients with BE. The test is not perfect and dysplasia can still be missed. Yet the likelihood of missing dysplasia is lower in VC compared to random biopsies, which appears to miss more cases of dysplasia/cancer. A potential recommendation to improve the diagnostic yield in dysplasia detection is to combine VC and RB. That is to say, if VC is performed for targeted biopsies, then the endoscopist should additionally obtain random biopsies, which appear to have an additive effect on dysplasia detection.
Supplementary Material
Supplemental Table 1: Assessment of Study Quality using QUADAS
Supplemental Table 2: sensitivity analysis to detect changes in estimated risk difference based on varying external correlation.
Search Strategy for Chromoendoscopy (CE) on Medline.
(Esophagoscopy[mesh] AND Image Enhancement[mesh]) OR “Acetic 1) Acid”[Mesh] OR “Indigo Carmine”[Mesh] OR “Methylene Blue”[Mesh] OR “Coloring Agents”[Mesh] OR “Acetic Acid”[tiab] OR “Indigo Carmine”[tiab] OR “Methylene Blue”[tiab] OR “chromoendoscopy”[tiab] OR “Coloring Agents”[tiab]
“Barrett Esophagus”[Mesh] OR “Esophageal Neoplasms”[Mesh] OR “Barrett Esophagus”[tiab] OR “Barrett's Esophagus”[tiab] OR (Esophag*[tiab] AND (neoplasm*[tiab] OR cancer*[tiab] OR dysplas*[tiab] OR carcinoma*[tiab] OR precancer*[tiab] OR metaplas*[tiab]))
Search Strategy for Virtual Chromoendoscopy (VE) on Medline.
“Barrett Esophagus”[Mesh] OR “Esophageal Neoplasms”[Mesh] OR “Barrett Esophagus”[tiab] OR “Barrett's Esophagus”[tiab] OR (Esophag*[tiab] AND (neoplasm*[tiab] OR cancer*[tiab] OR dysplas*[tiab] OR carcinoma*[tiab] OR precancer*[tiab] OR metaplas*[tiab]))
(Esophagoscopy[mesh] AND Image Enhancement[mesh]) OR “narrow band imaging”[tiab] OR “nbi”[tiab] OR “autofluorescen*”[tiab] OR “trimodal”[tiab] OR “Fujinon intelligent chromoendoscopy”[tiab] OR “virtual chromoendoscopy”[tiab])
Search Strategy for Chromoendoscopy (CE) on Embase.
‘Barrett esophagus’/exp OR ‘esophagus cancer’/exp OR ‘esophageal adenocarcinoma’/exp OR ‘esophagus tumor’/exp OR ‘squamous cell metaplasia’/exp
‘chromoendoscopy’/exp OR ‘methylene blue’/exp OR ‘indigo carmine’/exp OR ‘acetic acid’/exp OR ‘screening test’/exp OR ‘coloring agent’/exp OR chromoendoscopy: ab,ti OR methylene blue:ab,ti OR indigo carmine:ab,ti OR acetic acid:ab,ti OR screening test:ab,ti OR coloring agent:ab,ti
Search Strategy for Virtual Chromoendoscopy (VE) on Embase.
‘Barrett esophagus’/exp OR ‘esophagus cancer’/exp OR ‘esophageal adenocarcinoma’/exp OR ‘esophagus tumor’/exp OR ‘squamous cell metaplasia’/exp
‘chromoendoscopy’/exp OR ‘screening test’/exp OR chromoendoscopy:ab,ti OR screening test:ab,ti OR ‘narrow band imaging’/exp OR Fujinon intelligent chromoendoscopy:ab,ti OR ‘autofluorescence imaging’/exp OR ‘narrow band imaging’:ab,ti OR ‘autofluorescence imaging’:ab,ti
Acknowledgments
Dr. White's effort was supported in part by a NIDDK Career Development Award (K01 DK078154-04) and the Houston VA HSR&D Center of Excellence (HFP90-020). Dr. Sharma receives funding from Grant suport from Olympus, Cook and Takeda. Drs. Qumseya, Wang, Uzomba, Badie, and Parasa have no conflicts to report.
Abbreviations
- AI
advanced imaging
- BE
Barrett's esophagus
- CBE
Complete Barrett Excision
- CE
chromoendoscopy
- CI
confidence interval
- CLE
Confocal laser endomicroscopy
- EAC
esophageal adenocarcinoma
- FICE
Fujinon intelligent chromoendoscopy
- HGD
high grade dysplasia
- LGD
low-grade dysplasia
- NBI
Narrow band imaging
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- QUADAS
Quality Assessment of Diagnostic Accuracy Studies
- RB
random biopsies
- RD
risk difference
- VC
virtual chromoendoscopy
- WLE
white light endoscopy
Footnotes
Author Contributions: Qumseya, Wang, Badie, Uzomba,: a, b, c, d, e
Parasa: c
Sharma, Wolfsen, White, Wallace: a,b,d,e
a. conception and design,
b. analysis and interpretation of data,
c. drafting of the article,
d. critical revision of the article for important intellectual content,
e. final approval of the article
Conflicts of Interest: Drs. Wallace and Wolfsen receive research funding from Olympus and Ninepoint Medical. Dr. Wallace is also a consultant for Cosmo pharmaceuticals. Dr. Wolfsen receives research funding from Olympus and NinePoint Medical. Dr. Wolfsen also is a consultant for CSA Medical, Mauna Kea Tech, Covidien (formerly BARRx).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rastogi A, Puli S, El-Serag HB, et al. Incidence of esophageal adenocarcinoma in patients with Barrett's esophagus and high-grade dysplasia: a meta-analysis. Gastrointest Endosc. 2008;67(3):394–8. doi: 10.1016/j.gie.2007.07.019. Epub 2007/11/30. [DOI] [PubMed] [Google Scholar]
- 2.Drewitz DJ, Sampliner RE, Garewal HS. The incidence of adenocarcinoma in Barrett's esophagus: a prospective study of 170 patients followed 4.8 years. Am J Gastroenterol. 1997;92(2):212–5. [PubMed] [Google Scholar]
- 3.Sharma P, Falk GW, Weston AP, et al. Dysplasia and cancer in a large multicenter cohort of patients with Barrett's esophagus. Clin Gastroenterol Hepatol. 2006;4(5):566–72. doi: 10.1016/j.cgh.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Vizcaino AP, Moreno V, Lambert R, et al. Time trends incidence of both major histologic types of esophageal carcinomas in selected countries, 1973-1995. Int J Cancer. 2002;99(6):860–8. doi: 10.1002/ijc.10427. Epub 2002/07/13. [DOI] [PubMed] [Google Scholar]
- 5.Pohl H, Welch HG. The role of overdiagnosis and reclassification in the marked increase of esophageal adenocarcinoma incidence. J Natl Cancer Inst. 2005;97(2):142–6. doi: 10.1093/jnci/dji024. Epub 2005/01/20. [DOI] [PubMed] [Google Scholar]
- 6.van Blankenstein M, Looman CW, Hop WC, et al. The incidence of adenocarcinoma and squamous cell carcinoma of the esophagus: Barrett's esophagus makes a difference. Am J Gastroenterol. 2005;100(4):766–74. doi: 10.1111/j.1572-0241.2005.40790.x. Epub 2005/03/24. [DOI] [PubMed] [Google Scholar]
- 7.Brown LM, Devesa SS. Epidemiologic trends in esophageal and gastric cancer in the United States. Surg Oncol Clin N Am. 2002;11(2):235–56. doi: 10.1016/s1055-3207(02)00002-9. [DOI] [PubMed] [Google Scholar]
- 8.Cameron AJ, Carpenter HA. Barrett's esophagus, high-grade dysplasia, and early adenocarcinoma: a pathological study. Am J Gastroenterol. 1997;92(4):586–91. [PubMed] [Google Scholar]
- 9.Wang KK, Sampliner RE. Updated guidelines 2008 for the diagnosis, surveillance and therapy of Barrett's esophagus. Am J Gastroenterol. 2008;103(3):788–97. doi: 10.1111/j.1572-0241.2008.01835.x. [DOI] [PubMed] [Google Scholar]
- 10.Hirota WK, Zuckerman MJ, Adler DG, et al. ASGE guideline: the role of endoscopy in the surveillance of premalignant conditions of the upper GI tract. Gastrointest Endosc. 2006;63(4):570–80. doi: 10.1016/j.gie.2006.02.004. Epub 2006/03/28. [DOI] [PubMed] [Google Scholar]
- 11.Spechler SJ, Sharma P, Souza RF, et al. American Gastroenterological Association medical position statement on the management of Barrett's esophagus. Gastroenterology. 2011;140(3):1084–91. doi: 10.1053/j.gastro.2011.01.030. Epub 2011/03/08. [DOI] [PubMed] [Google Scholar]
- 12.Levine DS, Haggitt RC, Blount PL, et al. An endoscopic biopsy protocol can differentiate high-grade dysplasia from early adenocarcinoma in Barrett's esophagus. Gastroenterology. 1993;105(1):40–50. doi: 10.1016/0016-5085(93)90008-z. Epub 1993/07/01. [DOI] [PubMed] [Google Scholar]
- 13.Falk GW, Rice TW, Goldblum JR, et al. Jumbo biopsy forceps protocol still misses unsuspected cancer in Barrett's esophagus with high-grade dysplasia. Gastrointest Endosc. 1999;49(2):170–6. doi: 10.1016/s0016-5107(99)70482-7. [DOI] [PubMed] [Google Scholar]
- 14.Wang KK, Okoro N, Prasad G, et al. Endoscopic evaluation and advanced imaging of Barrett's esophagus. Gastrointest Endosc Clin N Am. 2011;21(1):39–51. doi: 10.1016/j.giec.2010.09.013. Epub 2010/11/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kara MA, Ennahachi M, Fockens P, et al. Detection and classification of the mucosal and vascular patterns (mucosal morphology) in Barrett's esophagus by using narrow band imaging. Gastrointest Endosc. 2006;64(2):155–66. doi: 10.1016/j.gie.2005.11.049. [DOI] [PubMed] [Google Scholar]
- 16.Pohl J, May A, Rabenstein T, et al. Computed virtual chromoendoscopy: a new tool for enhancing tissue surface structures. Endoscopy. 2007;39(1):80–3. doi: 10.1055/s-2006-945045. Epub 2007/01/26. [DOI] [PubMed] [Google Scholar]
- 17.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. Epub 2009/07/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kanwal F, White D. “Systematic Reviews and Meta-analyses” in Clinical Gastroenterology and Hepatology. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2012;10(11):1184–6. doi: 10.1016/j.cgh.2012.09.019. Epub 2012/09/25. [DOI] [PubMed] [Google Scholar]
- 19.Whiting P, Rutjes AW, Reitsma JB, et al. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol. 2003;3(25) doi: 10.1186/1471-2288-3-25. Epub 2003/11/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lim CH, Rotimi O, Dexter SP, et al. Randomized crossover study that used methylene blue or random 4-quadrant biopsy for the diagnosis of dysplasia in Barrett's esophagus. Gastrointest Endosc. 2006;64(2):195–9. doi: 10.1016/j.gie.2005.07.025. [DOI] [PubMed] [Google Scholar]
- 21.Camus M, Coriat R, Leblanc S, et al. Helpfulness of the combination of acetic acid and FICE in the detection of Barrett's epithelium and Barrett's associated neoplasias. World J Gastroenterol. 2012;18(16):1921–5. doi: 10.3748/wjg.v18.i16.1921. Epub 2012/05/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Curvers WL, Herrero LA, Wallace MB, et al. Endoscopic tri-modal imaging is more effective than standard endoscopy in identifying early-stage neoplasia in Barrett's esophagus. Gastroenterology. 2010;139(4):1106–14. doi: 10.1053/j.gastro.2010.06.045. Epub 2010/07/06. [DOI] [PubMed] [Google Scholar]
- 23.Curvers WL, van Vilsteren FG, Baak LC, et al. Endoscopic trimodal imaging versus standard video endoscopy for detection of early Barrett's neoplasia: a multicenter, randomized, crossover study in general practice. Gastrointest Endosc. 2011;73(2):195–203. doi: 10.1016/j.gie.2010.10.014. Epub 2010/12/21. [DOI] [PubMed] [Google Scholar]
- 24.Wolfsen HC, Crook JE, Krishna M, et al. Prospective, controlled tandem endoscopy study of narrow band imaging for dysplasia detection in Barrett's Esophagus. Gastroenterology. 2008;135(1):24–31. doi: 10.1053/j.gastro.2008.03.019. [DOI] [PubMed] [Google Scholar]
- 25.Horwhat JD, Maydonovitch CL, Ramos F, et al. A randomized comparison of methylene blue-directed biopsy versus conventional four-quadrant biopsy for the detection of intestinal metaplasia and dysplasia in patients with long-segment Barrett's esophagus. Am J Gastroenterol. 2008;103(3):546–54. doi: 10.1111/j.1572-0241.2007.01601.x. Epub 2007/11/01. [DOI] [PubMed] [Google Scholar]
- 26.Curvers WL, Singh R, Song LM, et al. Endoscopic tri-modal imaging for detection of early neoplasia in Barrett's oesophagus: a multi-centre feasibility study using high-resolution endoscopy, autofluorescence imaging and narrow band imaging incorporated in one endoscopy system. Gut. 2008;57(2):167–72. doi: 10.1136/gut.2007.134213. [DOI] [PubMed] [Google Scholar]
- 27.Fortun PJ, Anagnostopoulos GK, Kaye P, et al. Acetic acid-enhanced magnification endoscopy in the diagnosis of specialized intestinal metaplasia, dysplasia and early cancer in Barrett's oesophagus. Alimentary pharmacology & therapeutics. 2006;23(6):735–42. doi: 10.1111/j.1365-2036.2006.02823.x. [DOI] [PubMed] [Google Scholar]
- 28.Ragunath K, Krasner N, Raman VS, et al. A randomized, prospective cross-over trial comparing methylene blue-directed biopsy and conventional random biopsy for detecting intestinal metaplasia and dysplasia in Barrett's esophagus. Endoscopy. 2003;35(12):998–1003. doi: 10.1055/s-2003-44599. [DOI] [PubMed] [Google Scholar]
- 29.Niepsuj K, Niepsuj G, Cebula W, et al. Autofluorescence endoscopy for detection of high-grade dysplasia in short-segment Barrett's esophagus. Gastrointest Endosc. 2003;58(5):715–9. doi: 10.1016/s0016-5107(03)02018-2. [DOI] [PubMed] [Google Scholar]
- 30.Wo JM, Ray MB, Mayfield-Stokes S, et al. Comparison of methylene blue-directed biopsies and conventional biopsies in the detection of intestinal metaplasia and dysplasia in Barrett's esophagus: a preliminary study. Gastrointest Endosc. 2001;54(3):294–301. doi: 10.1067/mge.2001.115732. [DOI] [PubMed] [Google Scholar]
- 31.Canto MI, Setrakian S, Willis J, et al. Methylene blue-directed biopsies improve detection of intestinal metaplasia and dysplasia in Barrett's esophagus. Gastrointest Endosc. 2000;51(5):560–8. doi: 10.1016/s0016-5107(00)70290-2. [DOI] [PubMed] [Google Scholar]
- 32.Kara MA, Peters FP, Rosmolen WD, et al. High-resolution endoscopy plus chromoendoscopy or narrow-band imaging in Barrett's esophagus: a prospective randomized crossover study. Endoscopy. 2005;37(10):929–36. doi: 10.1055/s-2005-870433. [DOI] [PubMed] [Google Scholar]
- 33.Gossner L, Pech O, May A, et al. Comparison of methylene blue-directed biopsies and four-quadrant biopsies in the detection of high-grade intraepithelial neoplasia and early cancer in Barrett's oesophagus. Digestive and Liver Disease. 2006;38(10):724–9. doi: 10.1016/j.dld.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 34.Sharma P, Hawes RH, Bansal A, et al. Standard endoscopy with random biopsies versus narrow band imaging targeted biopsies in Barrett's oesophagus: a prospective, international, randomised controlled trial. Gut. 2012 doi: 10.1136/gutjnl-2011-300962. Epub 2012/02/09. [DOI] [PubMed] [Google Scholar]
- 35.Sharma P, Savides TJ, Canto MI, et al. The American Society for Gastrointestinal Endoscopy PIVI (Preservation and Incorporation of Valuable Endoscopic Innovations) on imaging in Barrett's Esophagus. Gastrointest Endosc. 2012;76(2):252–4. doi: 10.1016/j.gie.2012.05.007. Epub 2012/07/24. [DOI] [PubMed] [Google Scholar]
- 36.Olliver JR, Wild CP, Sahay P, et al. Chromoendoscopy with methylene blue and associated DNA damage in Barrett's oesophagus. Lancet. 2003;362(9381):373–4. doi: 10.1016/s0140-6736(03)14026-3. [DOI] [PubMed] [Google Scholar]
- 37.Ngamruengphong S, Sharma VK, Das A. Diagnostic yield of methylene blue chromoendoscopy for detecting specialized intestinal metaplasia and dysplasia in Barrett's esophagus: a meta-analysis. Gastrointest Endosc. 2009;69(6):1021–8. doi: 10.1016/j.gie.2008.06.056. Epub 2009/02/14. [DOI] [PubMed] [Google Scholar]
- 38.Mannath J, Subramanian V, Hawkey CJ, et al. Narrow band imaging for characterization of high grade dysplasia and specialized intestinal metaplasia in Barrett's esophagus: a meta-analysis. Endoscopy. 2010;42(5):351–9. doi: 10.1055/s-0029-1243949. Epub 2010/03/05. [DOI] [PubMed] [Google Scholar]
- 39.Admad NZ, Ahmed A. A meta-analysis of randomized controlled trials comparing methylene blue-directed biopsies with random biopsies in the surveillance of Barrett's esophagus. Esophagus. 7(4):207–13. [Google Scholar]
- 40.Chung A, Bourke MJ, Hourigan LF, et al. Complete Barrett's excision by stepwise endoscopic resection in short-segment disease: long term outcomes and predictors of stricture. Endoscopy. 2011;43(12):1025–32. doi: 10.1055/s-0030-1257049. Epub 2011/11/10. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1: Assessment of Study Quality using QUADAS
Supplemental Table 2: sensitivity analysis to detect changes in estimated risk difference based on varying external correlation.


