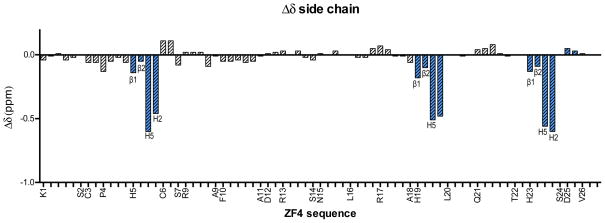
Figure 3.
The Δδ (δ(1-Peptide) – δ(apo-Peptide)) of amino acid side chain 1H chemical shifts (in ppm) plotted against peptide sequence of ZF4 at 15 ºC. For clarity, only the individual protons of the His residues are labeled while the remaining residues are labeled by the residue name at the corresponding Hβ/Hβ1. The remaining protons are plotted in order of closeness to the Hβ (e.g. K1 is plotted along the x-axis as Hβ1, β2, Hγ, Hδ, and Hε but labeled as K1 at the Hβ1 position). Fully labeled Δδ plots are available in SI Figure 5.The Δδ of the His protons are highlighted in blue for emphasis and labeled according to the Figure 1b. Coordination selectivity was observed by the significant effects of 1 on the His imidazole 1H resonances as compared to the other amino acid protons.