Abstract
Background
Sphingosine 1-phosphate (S1P) has been discovered to be a critical regulator of bone metabolism. Very recently, we found that higher circulating S1P levels were associated with higher rate of prevalent osteoporotic fracture in human.
Methods
This was a cross-sectional study of 16 patients who underwent hip replacement surgeries. Bone marrow fluids were obtained during hip surgeries, and the S1P levels were measured using a competitive enzyme-linked immunosorbent assay (ELISA) assay. Bone mineral densities (BMDs) at various skeletal sites were obtained using dual energy X-ray absorptiometry.
Results
Among 16 patients, 4 patients were undergone operations due to hip fractures, and the others were done by any other causes. Bone marrow S1P levels were significantly lower in patients with hip fractures than in those without, before and after adjusting for confounding factors (P=0.047 and 0.025, respectively). We failed to demonstrate significant associations between bone marrow S1P levels and any BMD values (γ=0.026-0.482, P=0.171-0.944).
Conclusions
In conjunction with our previous findings, these suggest that the effects of gradient between peripheral blood and bone marrow, but not S1P itself, may be the most critical on bone metabolism.
Keywords: Bone density, Osteoporotic fracture, Sphingosine 1-phosphate
INTRODUCTION
Sphingosine 1-phosphate (S1P) is a biologically active lysophospholipid which is produced by the metabolism of sphingolipid catalyzed by sphingosine kinase. S1P is involved in various cell activities including cell differentiation, apoptosis, proliferation, migration, and others.[1] The roles of S1P have recently drawn attention as S1P has been discovered to be a critical regulator of bone metabolism by many studies. First, S1P is released from osteoclasts and facilitates the proliferation, migration, and survival of osteoblasts by working as a coupling factor between osteoblasts and osteoclasts.[2-4] Second, S1P mediates bone destruction by inducing the formation of osteoclasts.[5] Third, S1P is more abundant in circulating blood than bone marrow cavity.[6-8] When the difference of S1P levels between those two increases, osteoclast precursors are limitedly present in bone marrow cavity, promoting bone resorption.[9-12] However, the role of S1P in humans and the relative importance of three mechanisms mentioned in the above have not been clarified yet. In relation to this, the authors of this study have recently reported that bone resorption is promoted and bone mineral density (BMD) decreases as plasma S1P levels become higher in the human body.[13] Moreover, the risk of vertebral fracture increases.[14] These previous findings imply that S1P is not only important to humans but also, involved in two mechanisms facilitating bone resorption by directly promoting bone resorption and maintaining constant concentration difference between circulation system and bone marrow cavity. In addition, the findings suggest that one of the bone resorption mechanisms is more important than the other and the bone formation-related mechanism. This investigation is a preliminary study investigating if which mechanism is more significantly involved between two bone resorption mechanisms. The study aims to identify the differences in S1P levels by collecting bone marrow in patients who underwent surgeries due to osteoporotic fractures and causes with other than osteoporotic fractures.
METHODS
1. Subjects
This study involved 16 patients who underwent hip surgery in the Department of Orthopedic Surgery, Asan Medical Center from July 2012 to September 2012. We excluded patients who took osteoporosis medications affecting bone metabolism for six months, and patients with diseases that develop secondary osteoporosis including hyperthyroidism and others. Osteoporotic hip fracture excluded high-impact fractures induced by car accident and others, and four subjects fell under this category (Table 1). The study was carried out after gaining the institutional review board (IRB) approval of Asan Medical Center and written consent form of all subjects.
Table 1.
Baseline characteristics of study subject
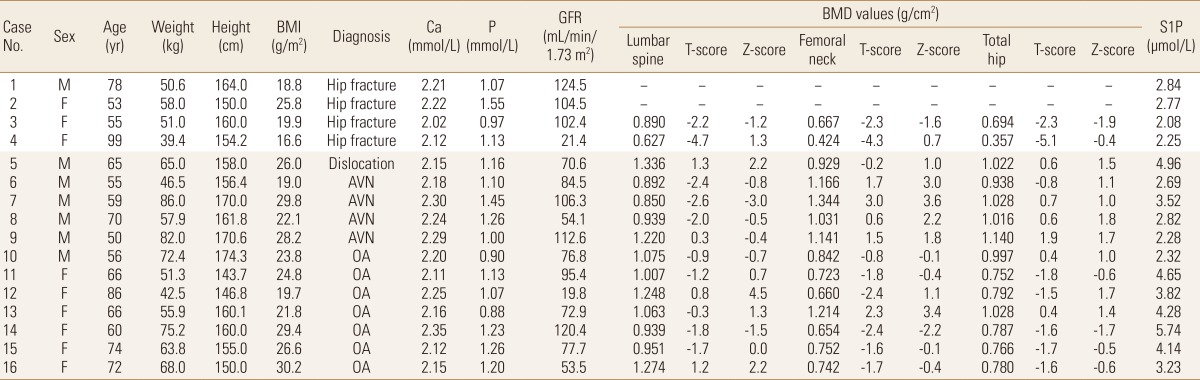
BMI, body mass index; Ca, calcium; P, phosphorus; GFR, glomerular filtration rate; BMD, bone mineral density; S1P, sphingosine 1-phsophate (mean values of 2 measurements); AVN, avascular necrosis; OA, osteoarthritis.
2. Anthropometric and biochemical measurements
The study investigated sex, age, body weight, and height of all subjects and identified their diagnosis at the time of operation. Body mass index (BMI) was obtained using weight and height. Bone marrow collected during surgery was stored in -70℃ deep freezer by dividing into 200 µL. Bone marrow samples were centrifuged at 3,000 rpm for 5 minutes at 4℃ to separate bone marrow fluids for the measurement of S1P levels (µmol/L). Centrifuged bone marrow fluids were kept at -70℃ before the measurement. S1P competitive enzyme-linked immunosorbent assay (ELISA) kit (Echelon Biosciences Inc., Salt Lake City, UT, USA) was used for the measurement of S1P levels. Minimum value was 0.06 µmol/L, and inter- and intra-measurement coefficients of variation were 6.4% and 6.1%, respectively. Bone marrow S1P level of each patient was measured twice and the average value was used for analysis. Serum calcium (mmol/L) and phosphorus levels (mmol/L) were measured by applying cresolphthalein comlexone method and phosphomolybdate ultraviolet (UV) method, respectively, using Toshiba 200FR Autoanalyzer (Toshiba Medical Systems Co., Ltd., Tokyo, Japan). Glomerular filtration rate (GFR) (mL/min/1.73 m2) was obtained using the Cockroft-Gault formula.[15] All coefficients of variation were found to be less than 3.5%.
3. Measurement of BMD
BMD (g/cm2) was measured in lumbar spine and proximal femur (femoral neck, total hip) using dual energy X-ray absorptiometry (Lunar Prodigy Advance, GE Lunar, Medison, WI, USA). Coefficients of variation were 0.67% in lumbar spine and 1.25% in femoral neck.
4. Statistical analysis
The differences of mean values between two groups were analyzed using an independent samples t-test, and the adjustment of confounding factors was performed with the analysis of covariance. Age and sex were included as confounding factors. Partial correlation analysis was performed to verify the association bewteen bone marrow S1P levels and BMDs, and three analysis models were established for the adjustment of confounding factors. Age and sex were included in Model 1, body weight and height were added in Model 2, and serum calcium, phosphorus levels and GFR were added in Model 3. P-values of less than 0.05 were considered to be statistically significant in all statistical analysis.
RESULTS
Nine patients (56%) were females among the total of 16 subjects. Three patients (75%) were females among 4 patients with osteoporotic hip fracture (Table 1). Among 12 patients who underwent operation due to causes other than osteoporotic hip fracture, 7 patients were osteoarthritis, 4 patients were avascular necrosis (AVN) of the femoral head, and a patient was hip dislocation. There were a male (25.0%) and three female (75.0%) patients with osteoporotic hip fractures. Mean age was 71.3±21.7 years, and BMI was 20.3±3.9 kg/m2. Control group comprised 6 males and females each. Mean age was 64.9±9.8 years, showing younger mean age (P=0.607), and BMI was 25.1±3.9 kg/m2, showing a higher tendency (P=0.050).
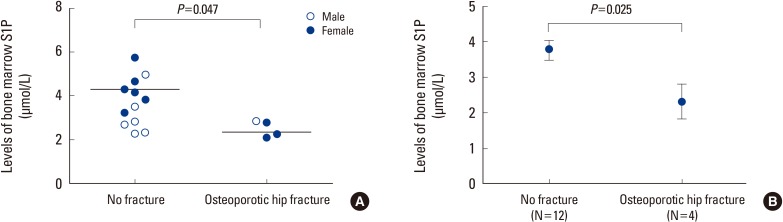
The average bone marrow S1P level of all subjects was found to be 3.40±1.10 µmol/L (range: 2.08-5.74 µmol/L). We compared bone marrow S1P levels of two groups according to the presence of osteoporotic hip fractures (Fig. 1). Bone marrow S1P levels were 2.48±0.38 µmol/L in patient group with osteoporotic hip fracture, and 3.70±1.09 µmol/L in control group. Bone marrow S1P level was significantly low in patients with osteoporotic hip fracture (P=0.047) (Fig. 1A). The difference between two groups was larger after adjusting for age and sex. Bone marrow S1P levels were 2.31±1.94 µmol/L in patient group with osteoporotic hip fracture and 3.76±1.10 µmol/L in control group, showing significance (P=0.025) (Fig. 1B). When we analyzed the correlation between bone marrow S1P level and BMD, statistically significant correlation was not observed after the adjustment of several confounding factors (γ=0.026-0.482, P=0.171-0.944) (Table 2).
Fig. 1.
Bone marrow sphingosine 1-phosphate (S1P) levels according to the osteoporotic hip fracture status were investigated (A) before and (B) after adjusting for confounding factors such as age and sex.
Table 2.
Associations of bone marrow sphingosine 1-phosphate levels with bone mineral density after adjusting for confounding factors
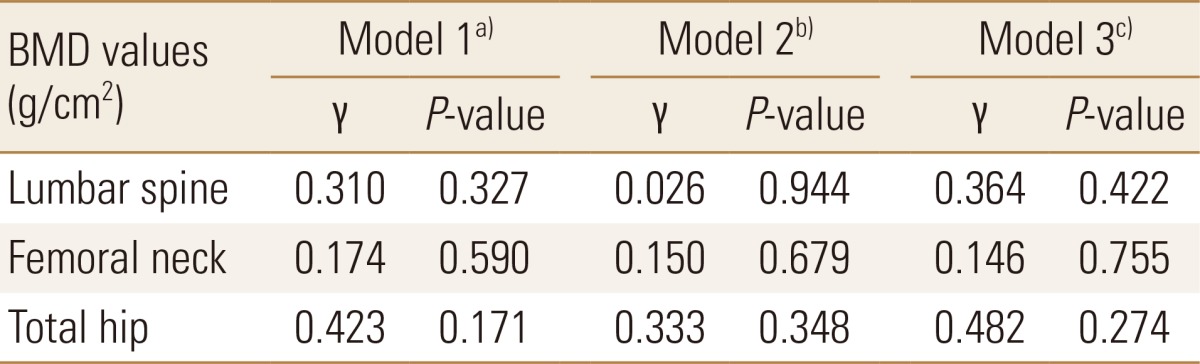
a)Model 1: adjusted for age and sex. b)Model 2: adjusted for age, sex, weight, and height. c)Model 3: adjusted for age, sex, weight, height, calcium, phosphorus, and glomerular filtration rate. BMD, bone mineral density.
DISCUSSION
This study identified that the bone marrow S1P levels were significantly lower in patients with osteoporotic hip fractures than in those without. No studies have been performed to verify the association between the presence of osteoporosis and osteoporotic fracture by clinically measuring S1P levels in the bone marrow of humans. The investigation is the first study to observe the positive effects on bone metabolism of high S1P levels in bone marrow, contradicting previous in vivo studies that suggested that S1P directly increases osteoclast differentiation and bone resorption.[1,5]
In a recent study, the authors have verified that BMDs decrease and bone resorption markers increase as plasma S1P levels become higher in all patients including men, and pre- and postmenopausal women.[5] Furthermore, the risk of osteoporotic vertebral fracture increased as well.[14] In contrast, this investigation found the reduction in the prevalence of osteoporotic fracture as bone marrow S1P levels increase. Thus, the risk of fracture may increase as S1P levels rise in peripheral blood. However, the risk of fracture may be reduced when bone marrow S1P level is higher. To sum up the above study results, S1P level is not directly involved in bone cells, instead bone resorption is accelerated as the concentration difference of S1P between peripheral blood and bone marrow cavity increases, supporting the hypothesis.[10]
High plasma S1P concentration is maintained as S1P is consistently released by vascular endothelial cells, red blood cells (RBCs), platelet, and others. On the other hand, concentration difference between plasma and tissue is formed by maintaining low S1P levels with constant breakdown catalyzed by intracellular S1P lyase or S1P phosphatase in each tissue including bone marrow.[9] The S1P receptor type 2 (S1PR2) expression of osteoclast precursors is dominant in peripheral blood with high S1P level, and it promotes the migration of osteoclast precursors to bone marrow by triggering the signaling pathways of G12/13-mediated Rho activation.[10] Once osteoclast precursors migrate into bone marrow, they are differentiated to osteoclasts. Consequently, attached osteoclasts on the surface of bones generated bone resorption. Therefore, the migration of osteoclast precursors to bone surface is accelerated as the difference of S1P level between peripheral blood and bone marrow is higher, leading to higher risk of fracture due to increased bone resorption.
This study failed to demonstrate significant association between bone marrow S1P levels and BMD values. However, the study involved a relatively small number of subjects, measuring BMDs only in 14 patients. Taking this fact into consideration, the results could not be interpreted that S1P is not taking a crucial role in bone metabolism. There is also limitation in generalizing the study results obtained from small sample size of 16 patients. Furthermore, the concentration difference between peripheral blood and bone marrow is more critical factor involved in osteoclast migration rather than S1P present in bone marrow. However, the study was unable to measure S1P levels in the blood of same patient. Therefore, this investigation is considered as a preliminary study suggesting the conduction of clinical trials in the future.
CONCLUSION
This study verified that bone marrow S1P level was lower in patient group with osteoporotic hip fractures compared with control group. These suggest that the difference of S1P levels between peripheral blood and bone marrow is critical on bone metabolism. Therefore, large-scale studies are thought to be crucial in the future.
Footnotes
No potential conflict of interest relevant to this article was reported.
This work was supported in part by the Grant for Young Investigator from the Korean Society of Bone Metabolism in 2007.
References
- 1.Rosen H, Goetzl EJ. Sphingosine 1-phosphate and its receptors: an autocrine and paracrine network. Nat Rev Immunol. 2005;5:560–570. doi: 10.1038/nri1650. [DOI] [PubMed] [Google Scholar]
- 2.Grey A, Xu X, Hill B, et al. Osteoblastic cells express phospholipid receptors and phosphatases and proliferate in response to sphingosine-1-phosphate. Calcif Tissue Int. 2004;74:542–550. doi: 10.1007/s00223-003-0155-9. [DOI] [PubMed] [Google Scholar]
- 3.Pederson L, Ruan M, Westendorf JJ, et al. Regulation of bone formation by osteoclasts involves Wnt/BMP signaling and the chemokine sphingosine-1-phosphate. Proc Natl Acad Sci U S A. 2008;105:20764–20769. doi: 10.1073/pnas.0805133106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roelofsen T, Akkers R, Beumer W, et al. Sphingosine-1-phosphate acts as a developmental stage specific inhibitor of platelet-derived growth factor-induced chemotaxis of osteoblasts. J Cell Biochem. 2008;105:1128–1138. doi: 10.1002/jcb.21915. [DOI] [PubMed] [Google Scholar]
- 5.Ryu J, Kim HJ, Chang EJ, et al. Sphingosine 1-phosphate as a regulator of osteoclast differentiation and osteoclast-osteoblast coupling. EMBO J. 2006;25:5840–5851. doi: 10.1038/sj.emboj.7601430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peest U, Sensken SC, Andréani P, et al. S1P-lyase independent clearance of extracellular sphingosine 1-phosphate after dephosphorylation and cellular uptake. J Cell Biochem. 2008;104:756–772. doi: 10.1002/jcb.21665. [DOI] [PubMed] [Google Scholar]
- 7.Zhao Y, Kalari SK, Usatyuk PV, et al. Intracellular generation of sphingosine 1-phosphate in human lung endothelial cells: role of lipid phosphate phosphatase-1 and sphingosine kinase 1. J Biol Chem. 2007;282:14165–14177. doi: 10.1074/jbc.M701279200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maeda Y, Seki N, Sato N, et al. Sphingosine 1-phosphate receptor type 1 regulates egress of mature T cells from mouse bone marrow. Int Immunol. 2010;22:515–525. doi: 10.1093/intimm/dxq036. [DOI] [PubMed] [Google Scholar]
- 9.Ishii M, Egen JG, Klauschen F, et al. Sphingosine-1-phosphate mobilizes osteoclast precursors and regulates bone homeostasis. Nature. 2009;458:524–528. doi: 10.1038/nature07713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ishii M, Kikuta J, Shimazu Y, et al. Chemorepulsion by blood S1P regulates osteoclast precursor mobilization and bone remodeling in vivo. J Exp Med. 2010;207:2793–2798. doi: 10.1084/jem.20101474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ishii M, Kikuta J. Sphingosine-1-phosphate signaling controlling osteoclasts and bone homeostasis. Biochim Biophys Acta. 2013;1831:223–227. doi: 10.1016/j.bbalip.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 12.Ishii T, Shimazu Y, Nishiyama I, et al. The role of sphingosine 1-phosphate in migration of osteoclast precursors; an application of intravital two-photon microscopy. Mol Cells. 2011;31:399–403. doi: 10.1007/s10059-011-1010-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee SH, Lee SY, Lee YS, et al. Higher circulating sphingosine 1-phosphate levels are associated with lower bone mineral density and higher bone resorption marker in humans. J Clin Endocrinol Metab. 2012;97:E1421–E1428. doi: 10.1210/jc.2012-1044. [DOI] [PubMed] [Google Scholar]
- 14.Kim BJ, Koh JM, Lee SY, et al. Plasma sphingosine 1-phosphate levels and the risk of vertebral fracture in postmenopausal women. J Clin Endocrinol Metab. 2012;97:3807–3814. doi: 10.1210/jc.2012-2346. [DOI] [PubMed] [Google Scholar]
- 15.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]