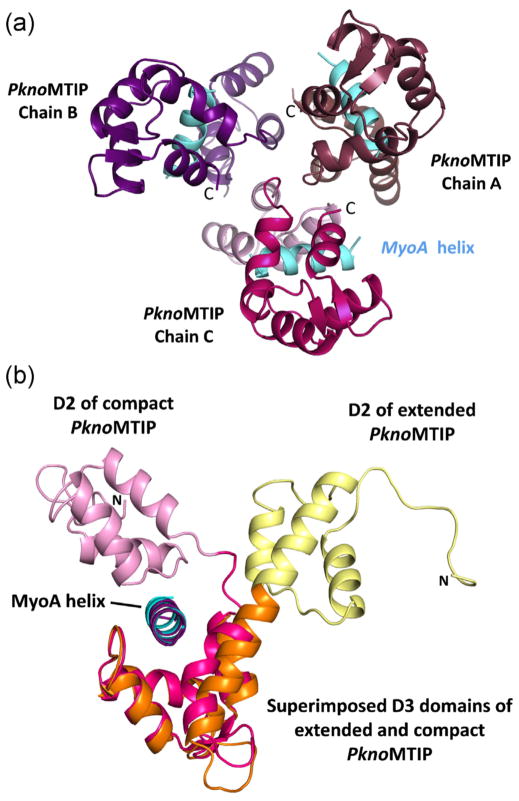
Fig. 1.
The structure of P. knowlesi MTIP•MyoA. (A) The three compact PknoMTIP•MyoA complexes in the asymmetric unit. The MyoA helices are depicted in sky blue. The D2 domain for each subunit is in lighter shade compared to the corresponding D3 domain. (B) Superposition of the D3 from the new compact PknoMTIP•MyoA complex (chain C, PknoMTIP-D2 is in pink, PknoMTIP-D3 in magenta and the MoyA helix in sky blue) onto the extended conformation in the PknoMTIP•MyoA complex (D2 is in yellow, D3 is in orange and MyoA helix is in deep purple) [9]. Residues D140-L204 from domain D3 were used to superpose the PknoMTIP-D3 domains. (For interpretation of the references to color in figure legend, the reader is referred to the web version of the article.)