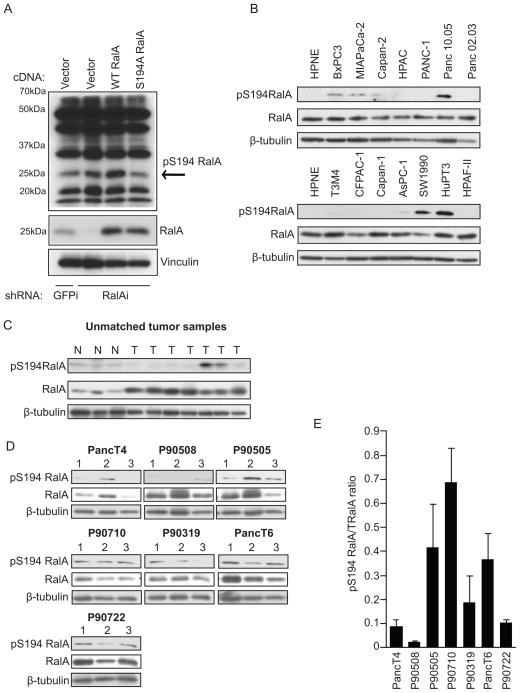
Figure 3.
RalA is phosphorylated on serine 194 in a subset of PDAC cell lines and human tumors. A, Western blot analysis of lysates from PANC-1 cells expressing GFP control or RalA-specific shRNA and RNAi-insensitive empty vector, wild type RalA, or phosphorylation-deficient (S194A) RalA using the rabbit site-specific (S194) phospho-serine RalA antibody. pS194 RalA band present at 25 kDa (indicated by the arrow) decreases in lysates expressing S194A RalA. B, Western blot analysis of lysates from HPNE and a panel of asynchronous PDAC cell lines using the S194 RalA phospho-specific antibody. Total RalA and β-tubulin are shown as loading controls. C, Western blot analysis of lysates from unmatched normal (N) and tumor (T) patient samples using the S194 RalA phospho-specific antibody. Total RalA and β-tubulin are shown as loading controls. D, Western blot analysis of PDX tumors treated with vehicle and harvested on treatment day 28 using S194 RalA phospho-specific antibody. Total RalA and β-tubulin are shown as loading controls. E, pS194 RalA to total RalA densitometry ratio quantitation of western blots in panel D. Data are representative of two or more independent experiments.