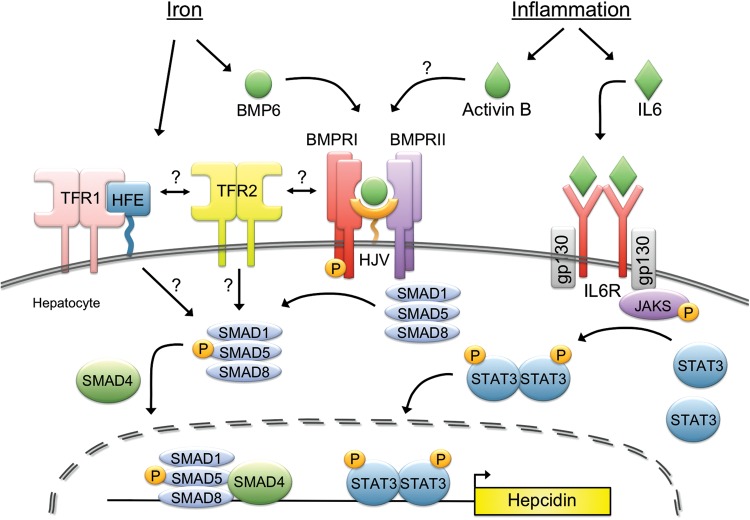
FIGURE 3:
Molecular regulation of hepcidin by iron and inflammation. Increased systemic iron stimulates the production of the ligand bone morphogenetic protein 6 (BMP6), which binds to the BMP Type I (ALK2/ALK3) and II (ACTRIIA) receptors, and the co-receptor HJV to stimulate phosphorylation of the SMAD1/5/8 intracellular signaling molecules. Phosphorylated SMAD 1/5/8 binds to SMAD4 and translocates to the nuclease to activate hepcidin transcription. The mechanism by which the hemochromatosis protein HFE and/or TFR2 regulate hepcidin expression is unknown but appears to involve an interaction with the BMP-SMAD signaling pathway. It has been proposed that an interaction between HFE and TFR1 is reduced under high iron conditions due to competitive binding of holotransferrin to TFR1. Displaced HFE could then associate with TFR2 and possibly the HJV-BMP receptor complex to regulate hepcidin. Inflammation also stimulates hepcidin production, in part via a canonical janus kinase (JAK)/signal transducer and activator of transcription (STAT) pathway in which inflammation increases interleukin 6 (IL6) binding to the IL6-receptor (IL6R) and thereby stimulating phosphorylation of JAKs and STAT3. Phosphorylated-STAT3 homodimers translocate to the nuclease and bind to the hepcidin promoter to stimulate hepcidin expression. Other mediators of inflammation and infection can also regulate hepcidin expression in this context (not shown). A mechanism of crosstalk between inflammatory signals and BMP signaling has been proposed in which inflammation induces activin B, which binds to BMP receptors to stimulate SMAD1/5/8 phosphorylation. SMADs and STAT3 may also interact at the level of the hepcidin promoter.