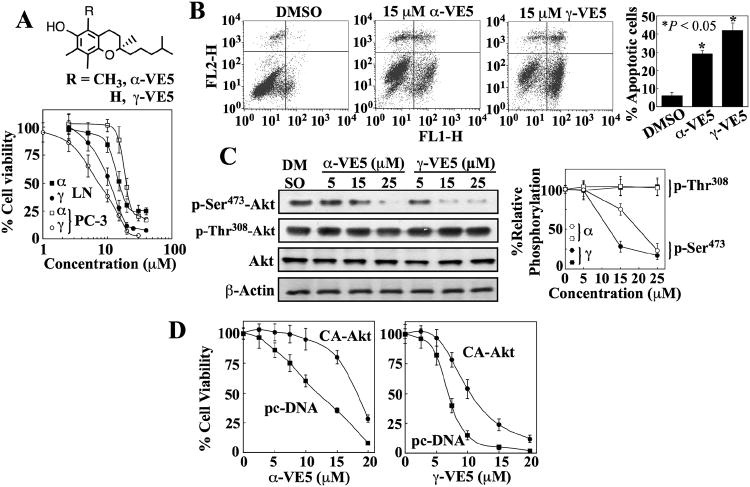
Fig. 3. Truncation of the aliphatic side chains of α- and γ-tocopherol enhances ability to induce apoptotic death through Akt inactivation.
(A) The chemical structures of α-VE5 and γ-VE5 (upper panel) and their effects on the viability of LNCaP (LN) and PC-3 cells (lower panel). Points, means; bars, ± S.D. (n = 6 replicates; data from a representative experiment). α, α-VE5; γ, γ-VE5. (B) Flow cytometric analysis of apoptosis in LNCaP cells measured by annexin V (FL1-H) and PI (FL2-H) staining after exposure to α- and γ-VE5. Representative dot plots and the percentages of cells that were apoptotic from 3 independent experiments are shown (means ± S.D.). *, P < 0.05 compared with the DMSO control (one-way ANOVA). (C) Western blot analysis of the effects of α- and γ-VE5 on the phosphorylation of Ser473- and Thr308-Akt (p-Ser473-Akt and p-Thr308-Akt) in LNCaP cells. A representative Western blot and relative phosphorylation of Akt at Ser473 and 308Thr from 3 independent experiments are shown (means ± S.D.). Densitometric signals from phosphorylated Akt were first normalized to that of total Akt, and then to that of β-actin. Replicate data are shown in fig. S3A. *, P < 0.05 compared to DMSO control (Kruskal-Wallis). (D) Protective effect of the ectopic expression of constitutively active Akt (CA-Akt) against the antiproliferative activities of α- and γ-VE5 in LNCaP cells. Control cells were transfected with the empty vector (pcDNA). Points, means; bars, ± S.D. (n = 6 replicates; data from a representative experiment).