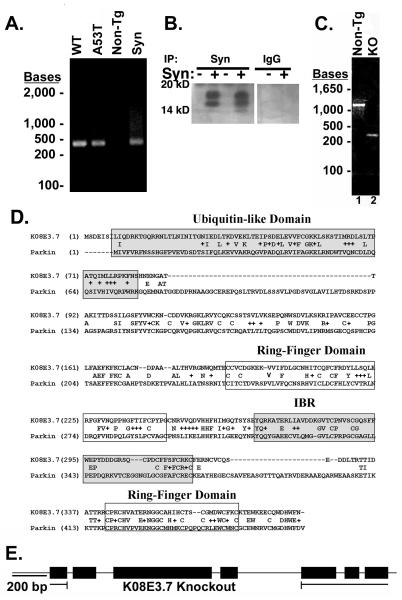
Figure 1. Verification of α-synuclein expression and knockout of K08E3.7 in transgenic C. elegans strains.
(A) PCR analysis of expressed wild type and A53T α-synuclein transgenes. 450 bp band is present in the wild type (WT) and A53T α-synuclein expressing lines (lanes 1 and 2) and co-migrates with a band amplified from α-synuclein cDNA (lane 4). The 450 bp α-synuclein-specific band is absent from the non-tg lane (lane 3). (B) Immunoprecipitation of α-synuclein demonstrates the presence of the α-synuclein protein (lanes 2 and 4, left panel). No synuclein is present in lysates from non-tg worms (lanes 1 and 3, left panel) nor in lysates immunoprecipitated with non-specific IgG (right panel). The doublet pattern of reactivity is often seen in immunoblots of α-synuclein. (C) PCR of genomic DNA across the KO8E3.7 gene yields a 1.37 Kb band for non-tg nematodes (lane 1) and a 0.26 Kb band for the K08E3.7 KO strain (lane 2). (D) The amino acid sequence alignment of K08E3.7 and human parkin. Exact amino acid matches are indicated with the identical amino acid in between the K08E3.7 and human parkin sequences; conservative amino acid differences are identified by a ‘+’. The N-terminal ubiquitin-like domain (shaded box – upper right), RING finger domains (boxed), in-between RING domain (shaded box) are indicated. (E) Diagram of the full-length K08E3.7 gene and the K08E3.7 gene containing the 1132 bp deletion from the K08E3.7 knockout.