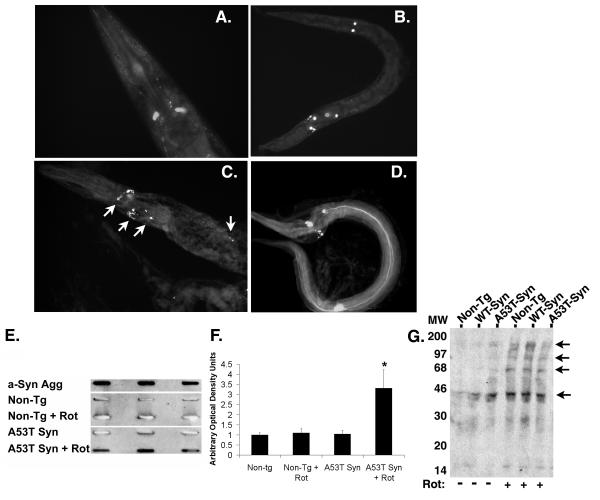
Figure 5. Increased fibrillogenisis in the A53T α-synuclein expressing line.
(A) GFP fluorescence present in dopaminergic neurons of BY200 worms after treatment with 25 μM rotenone for 2 days. (B) GFP fluorescence present in dopaminergic neurons of A53T α-synuclein worms after treatment with 25 μM rotenone for 2 days. (C & D) Staining with thioflavine S highlights additional fluorescence (arrows) present in A53T α-synuclein worms after treatment with 25 μM rotenone for 2 days, suggesting the presence of protein aggregates. (E) Slot blot analysis of non-tg and A53Tα-synculein expressing strain lysates using the Syn303 antibody that recognizes fibrillar α-synuclein. Row 1 – Recombinant aggregated α-synuclein (aged 1 month); Row 2 – non-tg strain; Row 3 – non-tg treated with rotenone (25 μM, 4 days); Row 4 – A53T α-synuclein expressing strain; Row 5 – A53T α-synuclein expressing strain treated with rotenone (25 μM, 4 days). Lysates were analyzed in triplicate. Aggregated α-synuclein was observed in the recombinant α-synuclein and in the A53T α-synuclein expressing strain treated with rotenone. (F) Quantification of slot blot analysis; (*) p<0.01 compared to non-tg. (G) Oxyblot analysis of total lysates from C. elegans treated with 25 μM rotenone for 2 days shows increased protein oxidation (arrows).