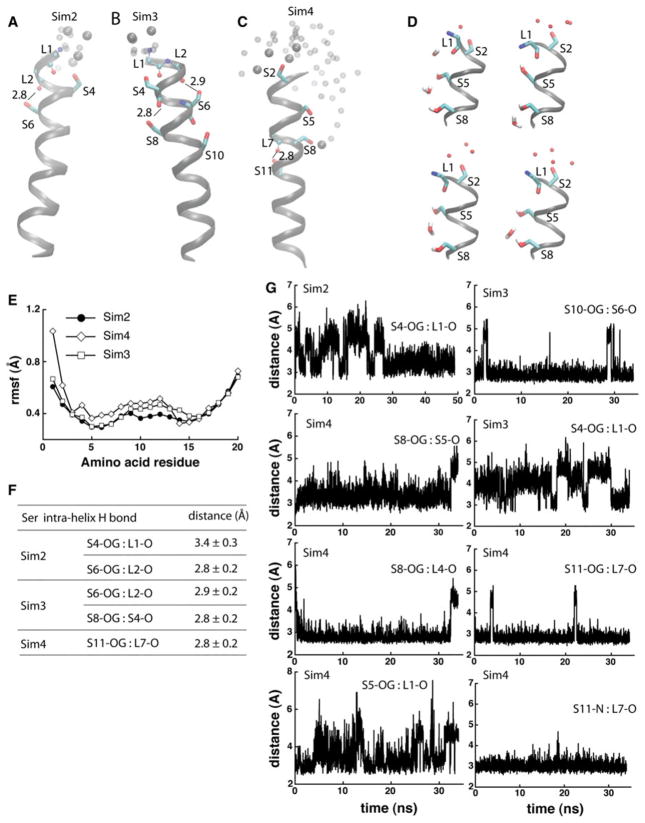
Fig. 4.
Dynamics and hydrogen bonding of Ser-containing poly-Leu peptides. a, b Coordinate snapshots from Sim2–Sim4 showing the peptide and specific amino acids at the end of the Sims. Ser side chains are shown as bonds; atoms of backbone groups involved in Ser hydrogen bonding are shown as small spheres. Oxygen atoms of water molecules within 6 Å from Ser groups are shown as van der Waals spheres for the snapshot at the of the Sims, and as small transparent spheres for 2 coordinate snapshots taken 2 and 1 ns before the end of the Sims, respectively. For simplicity, in panels a–c hydrogen are not shown. In Sim4, water molecules penetrate into the lipid bilayer, forming hydrogen bonds with S8 and/or S5. d Snapshots from the last ~1 ns segment of Sim4 illustrating hydrogen bonding between Ser side chains and water molecules; selected hydrogen atoms are depicted for examples of hydrogen bonding. e Rmsf (Å) computed from the last 10 ns of Sim2–Sim4. f Selected Ser hydrogen-bond distances measured during the last 10 ns segments of Sim2–Sim4. g Examples of time series of Ser hydrogen-bond distances in Sim2–Sim4