Abstract
Context
Severe sepsis, defined as infection complicated by acute organ dysfunction, occurs more frequently and leads to more deaths in black than in white individuals. The optimal approach to minimize these disparities is unclear.
Objective
To determine the extent to which higher severe sepsis rates in black than in white patients are due to higher infection rates or to a higher risk of acute organ dysfunction.
Design, Setting, and Participants
Analysis of infection-related hospitalizations from the 2005 hospital discharge data of 7 US states and infection-related emergency department visits from the 2003-2007 National Hospital Ambulatory Care Survey.
Main Outcome Measure
Age- and sex-standardized severe sepsis and infection hospitalization rates and the risk of acute organ dysfunction.
Results
Of 8 661 227 non–childbirth-related discharges, 2 261 857 were associated with an infection, and of these, 381 787 (16.8%) had severe sepsis. Black patients had a 67% higher age- and sex-standardized severe sepsis rate than did white patients (9.4; 95% confidence interval [CI], 9.3-9.5 vs 5.6; 95% CI, 5.6-5.6 per 1000 population; P<.001) and 80% higher standardized mortality (1.8, 95% CI, 1.8-1.9 vs 1.0, 95% CI, 1.0-1.1 per 1000 population; P<.001). The higher severe sepsis rate was explained by both a higher infection rate in black patients (47.3; 95% CI, 47.1-47.4 vs 34.0; 95% CI, 33.9-34.0 per 1000 population; incidence rate ratio, 1.39; P<.001) and a higher risk of developing acute organ dysfunction (age- and sex-adjusted odds ratio [OR],1.29; 95% CI, 1.27-1.30; P<.001). Differences in infection presented broadly across different sites and etiology of infection and for community- and hospital-acquired infections and occurred despite a lower likelihood of being admitted for infection from the emergency department (adjusted OR, 0.70; 95% CI, 0.64-0.76; P<.001). The higher risk of organ dysfunction persisted but was attenuated after adjusting for age, sex, comorbid conditions, poverty, and hospital effect (OR, 1.14; 95% CI, 1.13-1.16; P<.001). Racial disparities in infection and severe sepsis incidence and mortality rates were largest among younger adults (eg, the proportion of invasive pneumococcal disease occurring in adults <65 years was 73.9% among black patients vs 44.5% among white patients, P<.001).
Conclusion
Racial differences in severe sepsis are explained by both a higher infection rate and a higher risk of acute organ dysfunction in black than in white individuals.
SEVERE SEPSIS IS A BROAD CLINI-cal syndrome defined as infection complicated by acute organ dysfunction.1 It affects more than 750 000 US residents each year, with a hospital mortality of 28%.2 Epidemiological studies consistently report a higher incidence of severe sepsis among black than white patients.3 However, it is not known whether these disparities occur because of differences in susceptibility to infection or in the risk of developing acute organ dysfunction once infection has occurred. This distinction is important for developing interventions to reduce disparities. For example, if racial differences in severe sepsis are largely due to differences in the incidence of infection, efforts to reduce disparities should focus on community-based interventions, such as vaccination. On the other hand, if disparities are largely due to differences in the incidence of organ dysfunction, improving the management of black patients hospitalized with severe infections will be necessary.
Therefore hospital discharge data from 7 states in the United States were analyzed to determine the extent to which previously reported differences in severe sepsis incidence were due to a higher infection rate or a higher risk of acute organ dysfunction. Because differences could be due to different admission thresholds, we also analyzed national emergency department data.Whether infection and severe sepsis rates between the 2 races differed by age and comorbid conditions was determined to identify subgroups most amenable to interventions. In particular, the implications of age-related racial differences on vaccination were explored, focusing on invasive pneumococcal disease, because pneumococcal pneumonia is the most common cause of severe sepsis, and pneumococcal vaccination is the largest adult vaccination program for bacterial sepsis.4,5
METHODS
Design and Data Sources
We conducted retrospective, population-based analyses of hospitalized patients from the 2005 state hospital discharge databases of 7 US states to compare racial differences in infection and severe sepsis–related hospitalizations. We determined population-level estimates of infection and severe sepsis hospitalizations using 2005 population estimates that were based on the 2000 US census. We analyzed data from each state (Arizona, Florida, Massachusetts, Maryland, New Jersey, New York, and Texas) because they are large and diverse, represent a significant proportion (25%) of the US population, and maintain high-quality hospital discharge data, including information on self-reported race (<2% of patients elected not to report their race). We limited all analyses to non-Hispanic white and black patients because our prior work showed similar incidence rates of severe sepsis in Hispanic and non-Hispanic whites.3
To account for confounding due to different admission thresholds, we compared hospital admission rates for patients who presented to the emergency departments (EDs) with infection in the National Hospital Ambulatory Medical Care Survey (NHAMCS) from 2003 to 2007. Details of the NHAMCS survey methodology are described elsewhere.6 Briefly, the NHAMCS uses a 4-stage probability sample design to collect a nationally representative sample of ED visits in noninstitutional general and short-stay hospitals and excludes federal, military, and Veterans Affairs hospitals. Patient race is reported by hospital staff or obtained from medical records and is missing in approximately 12% of visits.
Case Definitions
In hospital discharge data, we identified hospitalizations for or complicated by bacterial and fungal infections based on International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes (eAppendix 1 and eTable 1 are available at http://www.jama.com). We used previously validated criteria to define severe sepsis as documented infection plus acute organ dysfunction.2 Consistent with severe sepsis entry criteria in prospective clinical studies,7 mechanical ventilation had to be listed for a patient to be assigned respiratory organ dysfunction. We also compared risk of acute organ dysfunction in the subset of patients with septicemia, as described previously.8
We used ICD-9-CM codes in primary and secondary diagnosis fields and Charlson criteria to identify presence of comorbid conditions.9 We excluded patients with human immunodeficiency virus (HIV) because compared with the general population, patients with HIV have higher infection rates and are more likely to develop severe sepsis. Furthermore, previous studies have shown that the prevalence of HIV varies by race.10,11 We used the same ICD-9-CM codes used for analyses of the hospital discharge data (eTable 1) to identify ED visits for bacterial or fungal infections in the NHAMCS data set.
Data Analyses
Using hospital discharge data, we determined racial differences in age, sex, and comorbidities for patients hospitalized with severe sepsis and infection using χ2 and t tests, as appropriate. We categorized frequency counts of hospitalizations and deaths due to severe sepsis, infections, and invasive pneumococcal disease in 5-year age increments.12 Unless otherwise stated, all rates reported are age and sex standardized.
We compared age- and sex-standardized population-based incidence rates of severe sepsis hospitalizations between the 2 races. We then estimated whether differences in severe sepsis incidence were explained by differences in incidence of infection-related hospitalizations and the risk of developing acute organ dysfunction, conditional on occurrence of an infection.
To compare differences in infection-related hospitalizations, we performed our primary analyses on all patients who were hospitalized for infection or whose hospitalization was complicated by an infection. Recognizing potential limitations of administrative data, we also conducted several sensitivity analyses to assess the robustness of our findings. First, because it is difficult with hospital discharge data to determine whether infection was present at admission or not, we used New York state data (which includes present-at-admission indicators for all diagnosis fields) to compare rates of infection at admission by race. Second, in the entire data set, we repeated our analyses for different sites and types of infection. Third, because less severe infections may be missed due to differences in access to care and different thresholds for hospital admission, we determined racial differences in postoperative infection rates which are less likely to be influenced by these factors. We assessed the risk of developing postoperative infections after common surgical procedures,13 including appendectomy, coronary artery bypass graft (CABG) surgery, carotid endarterectomy, colon resection, extremity bypass surgery, lower extremity joint replacement, hysterectomy, and lung resection using random-effects logistic regression. A list of the ICD-9-CM codes for these procedures and postoperative infection is provided online (eTable 2 available at http://www.jama.com).
We adjusted for age, sex, Charlson score, poverty, and hospital effect to compare the risk of hospital-acquired infection. We additionally accounted for confounding due to different hospital admission thresholds by comparing admission rates for infection-related ED visits between the 2 races using NHAMCS data. Finally, to ensure that higher rates of infection or severe sepsis in black patients were not simply because black patients were more likely to receive care at hospitals that reported higher infection rates, we compared the distribution of each race across all hospitals stratified by their reported infection rate.
We estimated age- and sex-adjusted population-based risks of severe sepsis. We then constructed serial random-effects logistic regression models on all infected patients to assess the risk of severe sepsis conditional on infection, adjusting for age, sex, poverty (proportion of white individuals below poverty as a measure of zip code–level economic privation),3 and comorbidity. We built models measuring comorbidity by the Charlson score and then by presence or absence of diabetes and chronic kidney disease, because these chronic diseases were more common among black patients. Each of these models was adjusted for clustering of patients by race at hospitals (hospital effect) and varying proportions of black patients by center using the decomposition method.14,15 These models yield 2 odds ratios (ORs), the within-hospital OR, which measures the association between race and severe sepsis risk conditional on being admitted to the same center, and the across-hospitals OR, which reflects the risk of severe sepsis across hospitals with varying proportions of black patients. Unless otherwise stated, we report the within-hospital ORs.
We explored the implications of age-related racial differences in infection-related hospitalizations on current guidelines for pneumococcal vaccination by estimating the proportion of patients hospitalized with invasive pneumococcal disease who would not be targeted by current vaccination guidelines. Vaccination is currently recommended to high-risk adults. High-risk adults include all persons aged 65 years or older, and younger persons who smoke or have chronic diseases. We did not include smoking status as a risk factor because reliable data about smoking history was not available.16
Database management and calculation of descriptive statistics were performed using Visual FoxPro and Excel (Microsoft Corporation, Redmond, Washington) and regression analyses were performed using Stata version 10.1 (Stata Corp, College Station, Texas). Racial distribution curves across hospitals ranked by overall infection rate were compared by Kolmogorov-Smirnov test. There was a greater than 90% power to detect a 0.5% difference in severe sepsis risk between the 2 races assuming 2-sided tests and an α of .05. The study was reviewed and exempted by the University of Pittsburgh institutional review board.
RESULTS
Of 8 661 227 non–childbirth-related hospitalizations, 2 261 857 (26.1%) infection-related hospitalizations were identified. Of these, 381 787 (16.8%) hospitalizations were also associated with acute organ dysfunction, and thus classified as severe sepsis (Figure 1). Among those hospitalized with infection and severe sepsis, respiratory infections were most common and occurred in a third of all cases (Table 1). Other common infections were genitourinary, abdominal, wound, and soft tissue infections, and bacteremia of unknown source. The distribution of type of infection was similar between the 2 races.
Figure 1.
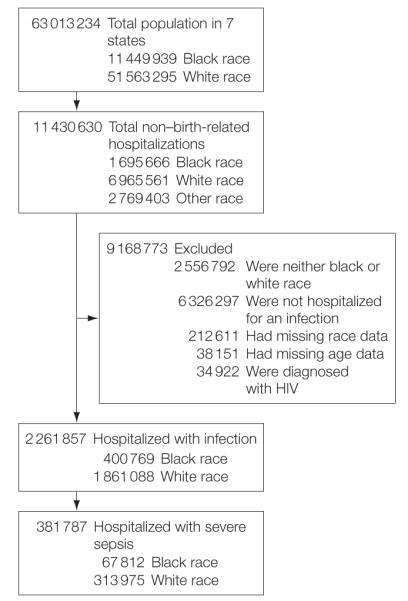
Overview of Analysis Cohort
HIV indicates human immunodeficiency virus.
Table 1.
Characteristics of Patients Hospitalized With Severe Sepsis and Infection
| Infection |
Severe Sepsis |
|||||
|---|---|---|---|---|---|---|
| Characteristic | Black Patients (n = 400 769) |
White Patients (n = 1 861 088) |
P Value |
Black Patients (n = 67 812) |
White Patients (n = 313 975) |
P Value |
| Age, mean, (median), y | 51.6 (54.0) | 63.1 (69.0) | <.001 | 62.0 (65.0) | 70.3 (74.0) | <.001 |
|
| ||||||
| Male sex, No. (%) | 161 597 (40.3) | 785 800 (42.2) | <.001 | 30 681 (45.2) | 152 401 (48.5) | <.001 |
|
| ||||||
| Underlying comorbidity, No.
(%) Pulmonary disease |
20 877 (5.2) | 194 903 (10.5) | <.001 | 5675 (8.4) | 44 841 (14.3) | <.001 |
|
| ||||||
| Neoplasm | 28 374 (7.1) | 167 714 (9.0) | <.001 | 7291 (10.8) | 38 042 (12.1) | <.001 |
|
| ||||||
| Chronic liver disease | 5195 (1.3) | 30 246 (1.6) | <.001 | 2242 (3.3) | 12 666 (4.0) | <.001 |
|
| ||||||
| Chronic kidney disease | 13 855 (3.5) | 48 712 (2.6) | <.001 | 4546 (6.7) | 17 979 (5.7) | <.001 |
|
| ||||||
| Diabetes mellitus | 94 600 (23.6) | 330 846 (17.8) | <.001 | 15 132 (22.3) | 48 734 (15.5) | <.001 |
|
| ||||||
| Peripheral vascular disease | 12 617 (3.2) | 60 432 (3.3) | .001 | 1931 (2.9) | 8241 (2.6) | .001 |
|
| ||||||
| Autoimmune disease | 8811 (2.2) | 38 452 (2.1) | <.001 | 1674 (2.5) | 4938 (1.6) | <.001 |
|
| ||||||
| Any comorbidity | 165 902 (41.4) | 785 445 (42.2) | <.001 | 33 950 (50.1) | 153 510 (48.9) | <.001 |
|
| ||||||
| Site of infection, No.
(%) Respiratory |
123 021 (30.7) | 641 123 (34.5) | <.001 | 23 807 (35.1) | 125 686 (40.0 | <.001 |
|
|
|
|||||
| Bacteremia, site unspecified | 39 189 (9.8) | 133 882 (7.2) | 16 287 (24.0) | 59 730 (19.0) | ||
|
|
|
|||||
| Genitourinary | 79 334 (19.8) | 321 181 (17.3) | 10 593 (15.6) | 46 670 (14.9) | ||
|
|
|
|||||
| Abdominal | 48 474 (12.1) | 273 168 (14.7) | 5130 (7.6) | 25 488 (8.1) | ||
|
|
|
|||||
| Device-related | 6100 (1.5) | 25 045 (1.4) | 893 (1.3) | 3365 (1.1) | ||
|
|
|
|||||
| Wound and soft tissue | 55 149 (13.8) | 270 676 (14.5) | 4823 (7.1) | 26 775 (8.5) | ||
|
|
|
|||||
| Central nervous system | 2591 (0.7) | 8106 (0.4) | 557 (0.8) | 1500 (0.5) | ||
|
|
|
|||||
| Endocarditis | 1681 (0.4) | 6202 (0.3) | 587 (0.9) | 2021 (0.6) | ||
|
|
|
|||||
| Other | 45 230 (11.3) | 181 705 (9.8) | 5135 (7.6) | 22 740 (7.2) | ||
|
| ||||||
| Type of
infection Fungal |
22 223 (5.6) | 91 607 (4.9) | <.001 | 4022 (5.9) | 18 116 (5.8) | .10 |
|
| ||||||
| Bacterial Gram positive |
44 571 (11.1) | 191 396 (10.3) | <.001 | 9441 (13.9) | 39 713 (12.7) | <.001 |
|
| ||||||
| Invasive pneumococcal | 1967 (0.5) | 9412 (0.5) | .23 | 698 (1.0) | 3121 (1.0) | .40 |
|
| ||||||
| MRSA | 11 716 (2.9) | 53 439 (2.9) | .07 | 1057 (1.6) | 5095 (1.6) | .23 |
|
| ||||||
| Gram negative | 49 768 (12.4) | 249 526 (13.4) | <.001 | 11 789 (17.4) | 57 470 (18.3) | <.001 |
|
| ||||||
| Etiology not known | 363 937 (90.8) | 1 715 460 (92.2) | <.001 | 60 306 (88.9) | 281 933 (89.8) | <.001 |
Abbreviation: MRSA, methicillin-resistant Staphylococcus aureus
Prehospitalization Characteristics
Table 1 compares characteristics of patients hospitalized with infections and severe sepsis. Among patients hospitalized with infections, black patients were younger than white patients (51.6 vs 63.1 years, P<.001). Small differences were observed in the overall burden of comorbid conditions (41.4% of black patients and 42.2% of white patients had at least 1 comorbid condition, P<.001). However, larger differences in the burden of certain comorbid conditions were noted. For example, 23.6% of black patients had diabetes and 3.5% had chronic kidney disease, whereas 17.8% of white patients had diabetes and 2.6% had chronic kidney disease (P<.001 for both comparisons). Pulmonary disease was more prevalent in white (10.5%) than in black patients (5.2%; P<.001). The higher prevalence of chronic kidney disease and diabetes persisted when analyses were stratified by occurrence of severe sepsis and was seen in patients older and younger than 65 years (Table 2).
Table 2.
Prevalence of Comorbidities in Patients Hospitalized With Infections and Severe Sepsis
| No. (%) of Patients With
Infection and Severe Sepsis | ||||||
|---|---|---|---|---|---|---|
| Black Patients |
White Patients |
|||||
| Characteristic | All | <65 y | ≥65 y | All | <65 y | ≥65 y |
| Infections | 400 769 | 266 734 | 134 035 | 1 861 088 | 815 096 | 1 045 992 |
|
| ||||||
| No comorbidities | 234 867 (58.6) | 180 030 (67.5) | 54 837 (40.9) | 1 075 643 (57.8) | 564 221 (69.2) | 511 422 (48.9) |
|
| ||||||
| Comorbidities | 165 902 (41.4) | 86 704 (32.5) | 79 198 (59.1) | 785 445 (42.2) | 250 875 (30.8) | 534 570 (51.1) |
|
| ||||||
| Chronic kidney disease | 13 855 (3.5) | 7427 (2.8) | 6428 (4.8) | 48 712 (2.6) | 11 949 (1.5) | 36 763 (3.5) |
|
| ||||||
| Diabetes mellitus | 94 600 (23.6) | 51 543 (19.3) | 43 057 (32.1) | 330 846 (17.8) | 120 954 (14.8) | 209 892 (20.1) |
|
| ||||||
| Severe sepsis | 67 812 | 33 480 | 34 332 | 313 975 | 95 223 | 218 752 |
|
| ||||||
| No comorbidities | 33 862 (49.9) | 17 863 (53.4) | 15 999 (46.6) | 160 465 (51.1) | 50 005 (52.5) | 110 460 (50.5) |
|
| ||||||
| Comorbidities | 33 950 (50.1) | 15 617 (46.6) | 18 333 (53.4) | 153 510 (48.9) | 45 218 (47.5) | 108 292 (49.5) |
|
| ||||||
| Chronic kidney disease | 4546 (6.7) | 2191 (6.5) | 2355 (6.9) | 17 979 (5.7) | 4064 (4.3) | 13 915 (6.4) |
|
| ||||||
| Diabetes mellitus | 15 132 (22.3) | 6942 (20.7) | 8190 (23.9) | 48 734 (15.5) | 15 312 (16.1) | 33 422 (15.3) |
Similarly, among those with severe sepsis, black patients were a mean 62.0 years vs white patients who were a mean 70.3 years (P<.001). Again, the overall burden of comorbid conditions was similar in both groups. However, the prevalence of certain comorbidities varied by race, similar to the pattern observed in patients with infections (Table 1 and Table 2).
Severe Sepsis and Infection Hospitalization Rates
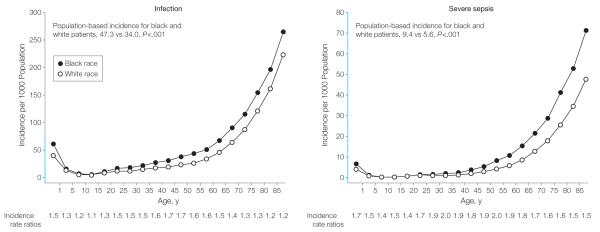
Black patients had a 67% higher severe sepsis hospitalization rate than did white patients (9.4; 95% confidence interval [CI], 9.3-9.5 vs 5.6; 95% CI, 5.6-5.6 per 1000 population; incidence rate ratio, 1.67, P<.001; incidence rate ratios across different age groups: 1.40-2.05, Figure 2). The difference in severe sepsis hospitalization rate was partly explained by a higher infection hospitalization rate (47.3; 95% CI, 47.1-47.4 vs 34.0; 95% CI, 33.9-34.0 per 1000 population; incidence rate ratio, 1.39; P<.001, incidence rate ratios across different age groups were 1.09 to 1.65, Figure 2). Black patients also had a higher hospitalization rate for septicemia (8.0; 95% CI, 7.9-8.1 vs 3.9; 95% CI, 3.9-4.0; P<.001).
Figure 2.
Population-Based Severe Sepsis and Infection Hospitalization Rates for Black Patients and White Patients in 7 US States, 2005
Differences were apparent across all age groups, but most pronounced in young adults between age 20 and 64 years, as reflected by the highest incidence rate ratios. The 67% higher severe sepsis rate in black patients was predominantly explained by a higher rate of infection hospitalizations. The y-axes, shown in blue, indicate the range of incidence from 0 to 80 per 1000 population. Class intervals include data for ages equal to the lower limit of each interval and up to but less than the upper limit.
Black patients consistently had higher infection-related hospitalization rates than did white patients both when using New York state data to analyze infections present at admission (38.4; 95% CI, 38.1-38.6 vs 28.2; 95% CI, 28.1-28.3 per 1000; incidence rate ratio, 1.36; P<.001) and when stratifying all infections in the entire data set by site (respiratory, septicemia, genitourinary, abdominal and wound and soft tissue) and type of infection (Table 3). An etiologic classification was available in approximately 25% of cases. Among all patients with infections, black patients had higher rates of Gram-positive and Gram-negative infections (Table 3).
Table 3.
Age- and Sex-Standardized Rates per 1000 Population for Different Infection Criteria, Sites, and Types of Infection-Related Hospitalizations
| Rate (95% Confidence
Interval) |
P Value |
||
|---|---|---|---|
| Type of Infection | Black Patients | White Patients | |
| Infection
criterion Infections present at admission |
38.4 (38.1-38.6) | 28.2 (28.1-28.3) | <.001 |
|
| |||
| Septicemia | 8.0 (7.9-8.1) | 3.9 (3.9-4.0) | <.001 |
|
| |||
| Site of
infection Respiratory |
15.0 (14.9-15.1) | 11.6 (11.5-11.6) | <.001 |
|
| |||
| Genitourinary | 9.2 (9.2-9.3) | 5.9 (5.9-5.9) | <.001 |
|
| |||
| Abdominal | 5.2 (5.2-5.3) | 5.1 (5.1-5.1) | <.001 |
|
| |||
| Wound and soft tissue | 6.1 (6.0-6.1) | 5.0 (5.0-5.0) | <.001 |
|
| |||
| Bacterial Gram positive |
5.1 (5.1-5.2) | 3.5 (3.5-3.5) | <.001 |
|
| |||
| Invasive pneumococcal | 0.22 (0.21-0.23) | 0.17 (0.17-0.18) | <.001 |
|
| |||
| MRSA | 1.2 (1.2-1.3) | 1.0 (1.0-1.0) | <.001 |
|
| |||
| Gram negative | 6.3 (6.3-6.4) | 4.5 (4.5-4.5) | <.001 |
|
| |||
| Fungal | 2.5 (2.5-2.6) | 1.7 (1.7-1.7)<.001 | |
Abbreviation: MRSA, methicillin-resistant Staphylococcus aureus.
Postoperative infections were most frequent after colon resection (4.9%), extremity bypass (2.3%), CABG (1.5%) and appendectomy (1.3%), while infection incidence rates after carotid endarterectomy, lower-extremity joint replacement, hysterectomy, and lung resection surgery were less than 1%. Compared with white patients (unadjusted OR,: 1.35; 95% CI, 1.26-1.45; P<.001), black patients were more likely to develop postoperative infections after these surgeries (adjusted OR, 1.27; 95% CI, 1.15-1.42; P<.001).
Higher hospital infection rates among black patients were not because they were more likely to be admitted with an infection. Indeed, the admission threshold for patients presenting to an ED with infection was higher for black than white patients (Table 4; age- and sex-adjusted OR of hospitalization among all patients who presented to the ED with an infection from 2003-2007 was 0.70, 95% CI, 0.64-0.76; P<.001). Similarly, higher infection rates were not because black patients were more likely to receive care at hospitals with higher recorded infection rates than white patients (P=.68).
Table 4.
Emergency Department Visits, Infection-Related Visits, and Proportion of Infection-Related Hospital Admissions From Hospitals Participating in the National Hospital Ambulatory Care Survey, 2003-2007
| Black Patients |
White Patients |
|||||
|---|---|---|---|---|---|---|
| No. (%) |
No. (%) |
|||||
| Year | No. of ED Visits |
Infection Diagnosisa |
Hospitalized for Infectionb |
No. of ED Visits |
Infection Diagnosisa |
Hospitalized for Infectionb |
| 2007 | 7561 | 1301 (17.2) | 129 (9.9) | 22 011 | 3430 (15.6) | 636 (18.5) |
|
| ||||||
| 2006 | 8695 | 1467 (16.9) | 153 (10.4) | 25 321 | 3948 (15.6) | 697 (17.7) |
|
| ||||||
| 2005 | 7737 | 1379 (17.8) | 129 (9.4) | 24 390 | 3793 (15.6) | 590 (15.6) |
|
| ||||||
| 2004 | 8860 | 1477 (16.7) | 148 (10.0) | 26 275 | 3798 (14.5) | 772 (20.3) |
|
| ||||||
| 2003 | 8424 | 1522 (18.1) | 158 (10.4) | 30 164 | 4599 (15.3) | 842 (18.3) |
|
| ||||||
| 2003-2007 | 41 277 | 7146 (17.3) | 717 (10.0) | 128 161 | 19 568 (15.3) | 3537 (18.1) |
Abbreviation: ED, emergency department.
Percentages in parentheses represent the proportion of infection-related visits of all ED visits
Percentages in parentheses represent the proportion of visits associated with hospital admission of all infection-related ED visits
Severe Sepsis and Organ Dysfunction
Conditional on an infection diagnosis, black patients across all age groups had a higher risk of developing acute organ dysfunction (age- and sex-adjusted OR, 1.29; 95% CI, 1.27-1.30; P<.001; Figure 3). Similarly, black patients with septicemia had a higher risk of organ dysfunction than white patients (age- and sex-adjusted OR, 1.38; 95% CI, 1.36-1.41; P<.001). Of the 381 787 patients hospitalized with severe sepsis, 296 552 (77.7%) had single organ dysfunction. Common organ dysfunctions were renal, respiratory, and cardiovascular failure. Seventy-eight percent of white patients had single organ dysfunction vs 76.0% of black patients (P<.001; Table 5). Blacks more frequently had renal (46.6% vs 43.8%, P<.001) and respiratory failure (34.4% vs 30.5%; P<.001) than white patients, while cardiovascular failure was more frequent in whites (25.5% vs 22.9%, P<.001).
Figure 3.
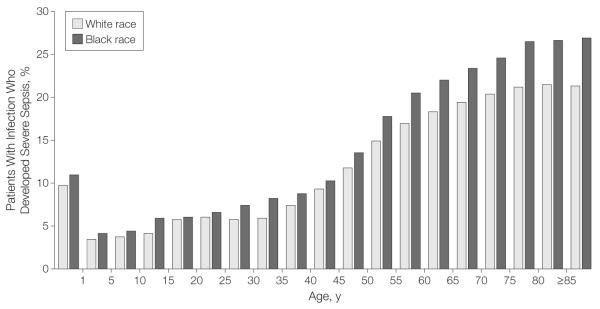
Risk of Severe Sepsis Conditional on Infection by Race
The additional risk of developing organ dysfunction conditional on infection was small. Class intervals include data for ages equal to the lower limit of each interval and up to but less than the upper limit.
Table 5.
Organ Dysfunction Stratified by Race Among Patients Hospitalized With Severe Sepsis
| No. (%) |
|||
|---|---|---|---|
| Black Patients (n = 67 812) |
White Patients (n = 313 975) |
P Value |
|
| No. of organ
dysfunctions 1 |
51 514 (76.0) | 245 038 (78.0) | <.001 |
|
| |||
| 2 | 12 459 (18.4) | 53 548 (17.1) | |
|
| |||
| 3 | 3214 (4.7) | 13 182 (4.2) | |
|
| |||
| ≥4 | 625 (0.9) | 2207 (0.7) | |
|
| |||
| Organ system
failing Respiratory |
23 348 (34.4) | 95 593 (30.5) | <.001 |
|
| |||
| Cardiovascular | 15 521 (22.9) | 80 119 (25.5) | <.001 |
|
| |||
| Renal | 31 598 (46.6) | 137 452 (43.8) | <.001 |
|
| |||
| Hematologic | 11 760 (17.3) | 57 627 (18.4) | <.001 |
|
| |||
| Central nervous system | 5463 (8.1) | 25 236 (8.0) | .87 |
|
| |||
| Hepatic | 924 (1.4) | 4679 (1.5) | .01 |
The higher risk of organ dysfunction among black patients persisted, although attenuated, when adjustment for poverty and hospital effect were included (OR, 1.15; 95% CI, 1.14-1.17). This attenuation was due in part because organ dysfunction was a more frequent complication of infection at hospitals that treated greater proportions of black patients (adjusted OR across hospitals, 1.15; 95% CI, 1.11-1.20; P<.001 for every 20% increase in proportion of black patients). Further inclusion of comorbidity, measured either by Charlson score (OR, 1.14; 95% CI, 1.13-1.16) or by preexisting kidney disease and diabetes (OR, 1.17; 95% CI, 1.15-1.18) yielded similar within-hospital odds of organ dysfunction among black patients.
Mortality
Mortality was higher among black than white patients hospitalized for infection and severe sepsis (age- and sex-adjusted OR, 1.23, 95% CI, 1.21-1.25 for black patients and 1.11; 95% CI, 1.08-1.14 for white patients; both P<.001). Furthermore, infection and severe sepsis mortality rates were 1.5-fold and 1.8-fold higher in black than in white patients (2.6; 95% CI, 2.6-2.7 vs 1.7; 95% CI, 1.7-1.7 per 1000 population and 1.8; 95% CI, 1.8-1.9 vs 1.0; 95% CI, 1.0-1.1 per 1000 population).
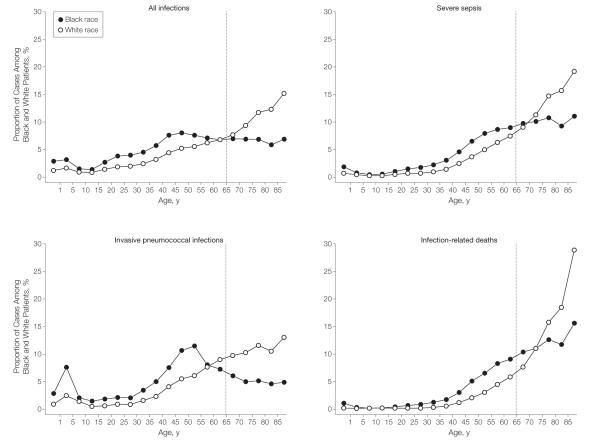
Differences in Infection-Related Hospitalization Rates by Age
Racial differences in infection-related hospitalizations were especially pronounced in those between the ages of 20 and 64 years, as illustrated by the highest incidence rate ratios in these age groups (range of incidence rate ratios, 1.7-2.0, Figure 2). Of black patients with infection-related hospitalizations, 266 734 (66.6%) occurred among those younger than 65 years, whereas 815 096 white patients (43.8%) were younger than 65 years (Figure 4). The higher proportion of infections persisted among black patients compared with white patients in this age group when we stratified analyses by site of infection (62.2% vs 33.8% for respiratory, 65.4% vs 42.3% for genitourinary, and 77.8% vs 61.3% for abdominal infections, respectively, all P<.001).
Similar to infection-related hospitalizations, a different distribution of severe sepsis cases across different age groups persisted between the 2 races (Figure 4). For example, 49.4% of severe sepsis cases involving black patients occurred among those younger than age 65 years.), whereas 69.7% of white patients with severe sepsis were 65 years and older. Infection- and severe sepsis-related deaths also affected more black than white patients younger than 65 years (Figure 4; 36% vs 18% of all infection-related deaths; 39% vs 22% of severe sepsis, respectively; all P<.001). Based on age- and sex-adjusted rates, young black patients were twice as likely to die of severe sepsis as young white patients (5.9; 95% CI, 5.7-6.0 vs 2.8; 95% CI, 2.8-2.9 per 10 000 population, incidence rate ratio, 2.1; P<.001).
Figure 4.
Infection and Severe Sepsis-Related Hospitalizations and Deaths
In contrast to whites, the majority of infections, severe sepsis cases, invasive pneumococcal infections, and deaths occurred in young black patients. For example, 74% of pneumococcal infections occurred in young black patients compared with 45% in young white patients younger than 65 years. Similarly, 49% vs 30% of severe sepsis cases, 74% vs 45% of invasive pneumococcal disease cases, and 36% vs 18% of infection related deaths occurred in black patients and white patients younger than 65 years, respectively. The gray line indicates 65 years of age.
Black patients had higher invasive pneumococcal disease hospitalization rates than did whites (22.3, 95% CI, 21.2-23.3 vs 17.1; 95% CI, 16.8-17.5 per 100 000 population; incidence rate ratio, 1.30; P<.001). Similarly 73.9% of invasive pneumococcal infections occurred in black patients younger than 65 years, whereas 44.5% occurred among white patients in this age group (P<.001, Figure 4). One in 4 pneumococcal infections occurred in black patients aged 18 to 65 years who had no underlying comorbidities and therefore would not have received pneumococcal vaccination based on current guidelines (23.6% in black patients vs 13.9% in white patients, P<.001). Invasive pneumococcal disease deaths substantially affected young black vs white patients younger than 65 years (57% vs 28%; P<.001).
COMMENT
In this large retrospective cohort study, we showed that the higher severe sepsis rates reported previously in black patients are explained by a higher likelihood of being hospitalized with infection and a higher risk of developing acute organ dysfunction. This finding was observed for both community- and hospital-acquired infections, consistent across different sites and etiologies of infection, and occurred despite the finding that black patients were less likely to be admitted when presenting to an ED with an infection. Thus, community-acquired interventions, such as vaccination and improved management of chronic diseases, and better management of those hospitalized with an infection to prevent organ dysfunction, are necessary to reduce disparities in severe sepsis.
The underlying mechanisms of racial disparities in infection and severe sepsis are poorly understood. A combination of differences in chronic disease burden, particularly subclinical disease, social and environmental factors, and genetic predisposition causing differences in the host immune response to infection likely contribute to the observed differences in infection and severe sepsis-related hospitalization rates.17-19 A higher prevalence of chronic kidney disease and diabetes was observed among black patients hospitalized for infection. Prior studies suggest that these conditions are more common among black patients in the US population.20,21 These differences may partly explain higher infection-related hospitalization rates among black patients, and better management of these conditions may reduce disparities. However, the higher infection rates were observed in those who did not report comorbidities, and these differences were observed as early as age 20 years. Furthermore, the differences in comorbidities did not explain higher risk of organ dysfunction among those hospitalized for infection. Differences in host immune response may explain these differences, as shown by recent studies suggesting polymorphisms in key proteins involved in the host response to infection may increase the susceptibility to severe infections and septic shock among people of African descent.19,22
The age-related differences in infection-related hospitalizations between the 2 races in our study occurred because black patients had more infections at a younger age, and they have lower life expectancy, therefore, leading to fewer elderly black individuals in the population.23 These findings have important public health implications for current vaccination guidelines. The number of cases of invasive pneumococcal disease missed by current vaccination guidelines was estimated to illustrate the implications of age differences in the incidence of infection-related hospitalizations and showed that 25% of invasive pneumococcal disease cases would be missed in blacks. Hence, earlier vaccination for black people, similar to the recommendation for earlier pneumococcal vaccination in Alaskan Natives and American Indians, should be considered to reduce disparities.24
Prior studies suggest that the quality of care for community-acquired pneumonia is lower at hospitals that treat larger proportions of blacks.15 A higher risk of organ dysfunction was observed at hospitals that provided care to larger proportion of black patients. These differences were independent of differences in demographic characteristics, poverty, and comorbidities. Thus, strategies that narrow quality gaps across hospitals in the care of infection and acute organ dysfunction could reduce racial disparities in severe sepsis. However, even after adjusting for differences across hospitals and baseline patient characteristics, black patients still had a residual increased risk of developing organ dysfunction and the mechanism for this finding is unclear.
Major strengths of this study are the large sample size and that the findings can be generalized to a large portion of the US population. In addition, several sensitivity analyses were conducted to account for potential confounders. Results were independent of sex, robust across different sources and etiologies of infections, and persisted after adjusting for poverty level. The analysis of national ED data suggests that the results may underestimate the difference in infection rates because black patients presenting to an ED with an infection are less likely to be admitted. Also excluded was the possibility that higher infection rates among black patients were simply because they were cared for more often at hospitals that report higher infection rates. The finding of higher infection rates among black patients persisted when restricted to analysis of patients with septicemia, to patients with infections present on admission, and to patients developing postoperative infections.
This study has important limitations. First, ICD-9-CM codes may not accurately identify infection and severe sepsis cases. The ICD-9-CM codes may underestimate certain infections25 and may overestimate severe sepsis cases by including those cases for which organ dysfunction preceded infection. However, coding errors are unlikely to differ by race within hospitals. In addition, this and other studies showed that the number of severe sepsis cases was similar when identified by ICD-9-CM codes and by prospective clinical data collection performed by trained data collectors.2,8 Second, social determinants such as household size, income, and educational status have been shown to be associated with infection burden in children and adults.26,27 Other social conditions such as smoking status, alcohol consumption, and nutritional status may also contribute to higher infection and severe sepsis rates among blacks. The influence of these factors was not determined because they are not coded reliably in administrative data sets.
In conclusion, higher severe sepsis rates among black patients are explained by both higher infection-related hospitalization rates and a higher risk of acute organ dysfunction. Reducing these racial disparities will require community-based interventions, such as vaccination, improved management of chronic diseases, and hospital-based interventions targeted especially to hospitals that serve large proportions of black patients. Current guidelines for pneumococcal vaccination, one of the largest and most effective strategies to prevent severe sepsis, do not target up to 25% of cases among blacks.
Supplementary Material
Acknowledgments
Funding/Support: Dr Mayr was supported by grant T32 HL007820-10, and Dr Yende was supported by grant K23GM083215 both from the National Institutes of Health.
Role of the Sponsor: The funding agencies had no role in the design and conduct of the study, collection, management, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript.
Footnotes
Author Contributions: Drs Mayr and Yende had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Mayr, Yende, Linde-Zwirble, Barnato, Angus Acquisition of data: Linde-Zwirble Analysis and interpretation of data: Mayr, Yende, Linde-Zwirble, Peck-Palmer, Barnato, Weissfeld, Angus Drafting of the manuscript: Mayr, Yende Critical revision of the manuscript for important intellectual content: Linde-Zwirble, Peck-Palmer, Barnato, Weissfeld, Angus Statistical analysis: Mayr, Yende, Weissfeld Obtained funding: Yende, Angus. Study supervision: Linde-Zwirble, Barnato, Angus.
Financial Disclosures: None reported.
Disclaimer: Dr Angus, contributing editor to JAMA, had no role in the evaluation of or decision to publish this article.
Online-Only Material: The eAppendix and eTables 1 and 2 are available at http://www.jama.com.
REFERENCES
- 1.Levy MM, Fink MP, Marshall JC, et al. SCCM/ESICM/ACCP/ATS/SIS.2001SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. CritCare Med. 2003;31(4):1250–1256. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 2.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29(7):1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Barnato AE, Alexander SL, Linde-Zwirble WT, Angus DC. Racial variation in the incidence, care, and outcomes of severe sepsis: analysis of population, patient, and hospital characteristics. Am J Respir Crit Care Med. 2008;177(3):279–284. doi: 10.1164/rccm.200703-480OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laterre PF, Garber G, Levy H, et al. PROWESS Clinical Evaluation Committee. Severe community-acquired pneumonia as a cause of severe sepsis: data from the PROWESS study. Crit Care Med. 2005;33(5):952–961. doi: 10.1097/01.ccm.0000162381.24074.d7. [DOI] [PubMed] [Google Scholar]
- 5.Jackson LA, Janoff EN. Pneumococcal vaccination of elderly adults: new paradigms for protection. Clin Infect Dis. 2008;47(10):1328–1338. doi: 10.1086/592691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. [Accessed April 13, 2010];National Hospital Ambulatory Care Survey. http://www.cdc.gov/nchs/ahcd/about_ahcd.htm.
- 7.Angus DC, Birmingham MC, Balk RA, et al. E5 murine monoclonal antiendotoxin antibody in gram-negative sepsis: a randomized controlled trial. E5 Study Investigators. JAMA. 2000;283(13):1723–1730. doi: 10.1001/jama.283.13.1723. [DOI] [PubMed] [Google Scholar]
- 8.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348(16):1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 9.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 10.Reade MC, Angus DC. Epidemiology of Sepsis and non-infectious SIRS. In: Cavaillon J-M, Adrie C, editors. Sepsis and Non-infectious Systemic Inflammation: From Biology to Critical Care. Wiley-Blackwell; Weinheim: 2009. [Google Scholar]
- 11.Mrus JM, Braun L, Yi MS, Linde-Zwirble WT, Johnston JA. Impact of HIV/AIDS on care and outcomes of severe sepsis. Crit Care. 2005;9(6):R623–R630. doi: 10.1186/cc3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carriere KC, Roos LL. Comparing standardized rates of events. Am J Epidemiol. 1994;140(5):472–482. doi: 10.1093/oxfordjournals.aje.a117269. [DOI] [PubMed] [Google Scholar]
- 13.Liu JH, Etzioni DA, O’Connell JB, et al. Inpatient surgery in California: 1990-2000. Arch Surg. 2003;138(10):1106–1111. doi: 10.1001/archsurg.138.10.1106. [DOI] [PubMed] [Google Scholar]
- 14.Localio AR, Berlin JA, Ten Have TR, Kimmel SE. Adjustments for center in multicenter studies: an overview. Ann Intern Med. 2001;135(2):112–123. doi: 10.7326/0003-4819-135-2-200107170-00012. [DOI] [PubMed] [Google Scholar]
- 15.Mayr FB, Yende S, D’Angelo G, et al. Do hospitals provide lower quality of care to black patients for pneumonia? Crit Care Med. 2010;38(3):759–765. doi: 10.1097/CCM.0b013e3181c8fd58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jollis JG, Ancukiewicz M, DeLong ER, Pryor DB, Muhlbaier LH, Mark DB. Discordance of databases designed for claims payment versus clinical information systems: implications for outcomes research. Ann Intern Med. 1993;119(8):844–850. doi: 10.7326/0003-4819-119-8-199310150-00011. [DOI] [PubMed] [Google Scholar]
- 17.Braveman PA, Cubbin C, Egerter S, et al. Socioeconomic status in health research: one size does not fit all. JAMA. 2005;294(22):2879–2888. doi: 10.1001/jama.294.22.2879. [DOI] [PubMed] [Google Scholar]
- 18.Khor CC, Chapman SJ, Vannberg FO, et al. A Mal functional variant is associated with protection against invasive pneumococcal disease, bacteremia, malaria and tuberculosis. Nat Genet. 2007;39(4):523–528. doi: 10.1038/ng1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferwerda B, Alonso S, Banahan K, et al. Functional and genetic evidence that the Mal/TIRAP allele variant 180L has been selected by providing protection against septic shock. Proc Natl Acad Sci U S A. 2009;106(25):10272–10277. doi: 10.1073/pnas.0811273106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brancati FL, Whittle JC, Whelton PK, Seidler AJ, Klag MJ. The excess incidence of diabetic end-stage renal disease among blacks: a population-based study of potential explanatory factors. JAMA. 1992;268(21):3079–3084. [PubMed] [Google Scholar]
- 21.O’Brien TR, Flanders WD, Decoufle P, Boyle CA, DeStefano F, Teutsch S. Are racial differences in the prevalence of diabetes in adults explained by differences in obesity? JAMA. 1989;262(11):1485–1488. [PubMed] [Google Scholar]
- 22.Ferwerda B, McCall MB, Alonso S, et al. TLR4 polymorphisms, infectious diseases, and evolutionary pressure during migration of modern humans. Proc Natl Acad Sci U S A. 2007;104(42):16645–16650. doi: 10.1073/pnas.0704828104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wong MD, Shapiro MF, Boscardin WJ, Ettner SL. Contribution of major diseases to disparities in mortality. N Engl J Med. 2002;347(20):1585–1592. doi: 10.1056/NEJMsa012979. [DOI] [PubMed] [Google Scholar]
- 24.Morse DL, Pikering LK, Baker CJ, et al. Advisory Committee on Immunization Practices. Recommended adult immunization schedule: United States, 2009. Ann Intern Med. 2009;150(1):40–44. doi: 10.7326/0003-4819-150-1-200901060-00008. [DOI] [PubMed] [Google Scholar]
- 25.Hsu LY, Koh TH. Use of ICD-9 diagnosis codes in epidemiologic surveys may significantly underestimate the incidence and impact of invasive pneumococcal disease locally. Singapore Med J. 2008;49(5):441. [PubMed] [Google Scholar]
- 26.Dowd JB, Zajacova A, Aiello A. Early origins of health disparities: burden of infection, health, and socioeconomic status in US children. Soc Sci Med. 2009;68(4):699–707. doi: 10.1016/j.socscimed.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zajacova A, Dowd JB, Aiello AE. Socioeconomic and race/ethnic patterns in persistent infection burden among US adults. J Gerontol A Biol Sci Med Sci. 2009;64(2):272–279. doi: 10.1093/gerona/gln012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.