Abstract
Sarcoidosis is a multisystem, granulomatous disease that most often affects the lungs. The clinical course is highly variable; many patients undergo spontaneous remission, but up to a third of patients progresses to a chronic disease course. The development of pulmonary fibrosis (PF) in a subset of patients with chronic disease has a negative impact on morbidity and mortality. While sarcoidosis-associated PF can be progressive, it is often referred to as “burnt out” disease, a designation reflecting inactive granulomatous inflammation. The immune mechanisms of sarcoidosis-associated PF are not well understood. It is not clear if fibrotic processes are active from the onset of sarcoidosis in predisposed individuals, or whether a profibrotic state develops as a response to ongoing inflammation. Transforming growth factor β (TGF-β) is an important profibrotic cytokine, and in sarcoidosis, distinct genotypes of TGF-β have been identified in those with PF. The overall cytokine profile in sarcoidosis-associated PF has not been well characterized, although a transition from a T helper 1 to a T helper 2 signature has been proposed. Macrophages have important regulatory interactions with fibroblasts, and the role of alveolar macrophages in sarcoidosis-associated PF is a compelling target for further study. Elucidating the natural history of sarcoidosis-associated PF will inform our understanding of the fundamental derangements, and will enhance prognostication and the development of therapeutic strategies.
Sarcoidosis is a complex, granulomatous disease of unknown etiology. Many organ systems can be affected, although lung involvement is most common, occurring in greater than 75% of all patients. The clinical outcomes in sarcoidosis are highly variable. While more than half of patients undergo remission with no significant residual morbidity, a subset of patients develops chronic disease. By convention, chronic sarcoidosis is disease activity lasting at least 2 years.1 Chronic sarcoidosis is itself a heterogenous category, which subsumes a variety of types of organ damage, including pulmonary fibrosis (PF). Immunosuppression is variably effective in controlling disease activity. There is no cure for sarcoidosis, although lung transplantation can be life saving for those with severe lung involvement.2
Pulmonary fibrosis occurs in up to 20% of patients with sarcoidosis and is of important consequence: it is associated with increased morbidity, a higher risk of pulmonary hypertension, the need for lung transplantation, and increased mortality.3-8 We herein summarize the evaluation of sarcoidosis-associated PF, and review the immunopathologic underpinnings as they are currently understood. We highlight the recognized differences between nonfibrotic and fibrotic pulmonary sarcoidosis, and between idiopathic pulmonary fibrosis (IPF) and sarcoidosis-associated PF. Microscopic fibrosis, representing a normal healing response to inflammation, may be evident on sarcoid histology. When small and stable foci of fibrosis do not result in significant radiographic changes or functional impairment, they likely do not affect the clinical course of sarcoidosis. For the purpose of this review, sarcoidosis-associated PF refers to an extensive fibrotic process which results in clinical impairment.
RADIOGRAPHIC PATTERNS OF SARCOIDOSIS-ASSOCIATED PF
The diagnosis of sarcoidosis-associated PF is established by radiographic imaging. Historically, Scadding chest x-ray stages, representing patterns of parenchymal and hilar changes, were widely used to qualify the extent of pulmonary sarcoidosis. Volume loss, lung and hilar distortion, and/or fibrocystic changes define fibrotic sarcoidosis and Scadding stage IV. In recent years, high resolution computed tomography (HRCT) has emerged as a more sensitive modality by which to identify fibrotic changes9,10 Compared with chest x-ray stages, HRCT findings in sarcoidosis correlate better with pulmonary function and long-term prognoses.11 However, similar to finding scant fibrosis on histology, small areas of fibrosis on HRCT may be physiologically incidental, and pathologically distinct from an extensive fibrotic process. The lack of standardized diagnostic criteria, radiographic or otherwise, to define clinically relevant pulmonary fibrosis in sarcoidosis has limited our ability to classify and study this phenotype.
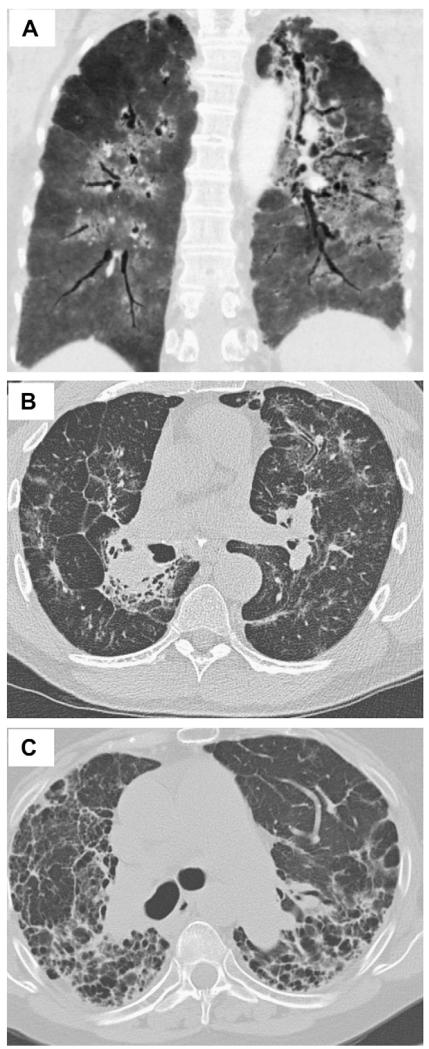
Three HRCT patterns of sarcoidosis-associated PF have been described: bronchial distortion, diffuse linear, and honeycombing fibrosis (Fig. 1).11 Bronchial distortion is marked by bronchial dilatation and angulation. A central location may predominate, or the distribution of bronchial changes may be diffuse. The extent to which bronchial distortion represents simple traction bronchiectasis versus damage to airway architecture from sarcoid inflammation is not known. As sarcoid inflammation occurs primarily along lymphatic channels, which are dense within bronchovascular tracts, bronchial distortion is the most common HRCT pattern of sarcoidosis-associated PF.11,12 The presence of scattered peripheral lines, translobular lines, and/or septal reticulation defines the second HRCT pattern of diffuse linear fibrosis. This pattern is the result of sarcoid inflammation within the lymphatics that course through interlobular septa. Finally, honeycombing fibrosis defines the third radiographic pattern of sarcoidosis-associated PF. Honeycomb cysts occur at the distal airway and alveolar level. This is the least common HRCT pattern, observed in less than half of patients with sarcoidosis-associated PF. It is worth noting that while one pattern may predominate in a given patient, many patients have an overlap of fibrotic patterns.11
Fig 1.
CT images demonstrate various patterns of pulmonary fibrosis in sarcoidosis. A, Honeycombing fibrosis, marked by innumerable cysts in both lungs. B, Diffuse linear fibrosis pattern, marked by residual areas of septal thickening. C, Bronchial distortion, marked by bronchial dilation and angulation.
CLINICAL FINDINGS IN SARCOIDOSIS-ASSOCIATED PF
Consistent with findings in other fibrotic lung conditions, pulmonary function tests in sarcoidosis-associated PF often reveal restriction. However, given the peribronchial distribution of lesions in sarcoidosis, obstruction can also be evident, and a mixed pattern of restriction and obstruction is common.11,13 Radiographic patterns may correlate with clinical outcomes. Patients with bronchial distortion may suffer from chronic bronchiectasis symptoms.4 Aspergillus mycetoma can develop within lung cavities and fibrocystic lesions, and are an important risk factor for hemoptysis, which can be severe. In addition, pre-existing mycetoma are associated with worse clinical outcomes in patients who undergo lung transplantation.14 Rarely, spontaneous pneumothorax complicates the course of fibrocystic sarcoidosis.4 Diffuse septal reticulation, a form of diffuse linear fibrosis, is associated with a higher risk of pulmonary hypertension.11 While pulmonary hypertension in sarcoidosis can be multifactorial, venous obstruction in the setting of extensive septal fibrosis has been proposed as an important mechanism.15 The development of pulmonary hypertension portends a particularly grave prognosis in sarcoidosis.14
The extent to which sarcoidosis-associated PF is progressive is not known.6,16 Further, in those with evident progression, it is not known if the clinical course is one of continuous or intermittent decline. There is no evidence that treatment in the acute phase of disease prevents progression to chronic sarcoidosis, or that immunosuppression alters the development or the course of sarcoidosis-associated PF.17,18 In patients with truly “burnt out” disease, a reference to the state of granulomatous inflammation, immunosuppression is unlikely to be of clinical benefit, and in our opinion should be withheld.19 However, while fibrosis is an irreversible finding, treatment may be beneficial when there is evidence of associated active inflammation. The presence of nodules and/or masses on thoracic imaging, in addition to other clinical indices of inflammation, such as elevated C-reactive protein or angiotensin-converting enzyme levels, can reflect a state of active granulomatous disease.11,20 It remains possible yet unproven that immunosuppression in those with signs of both fibrosis and active granulomatous inflammation may alter the long-term disease trajectory and associated clinical outcomes.
FIBROSIS HISTOPATHOLOGY IN SARCOIDOSIS-ASSOCIATED PF
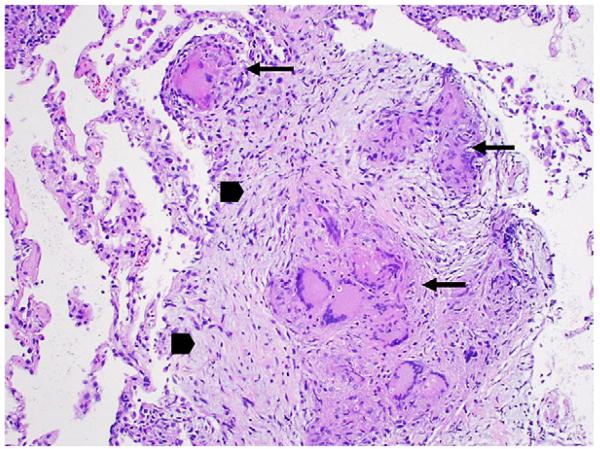
Fibrosis is the result of fibroblast activation and proliferation, with the subsequent production and deposition of collagen. In sarcoidosis these events occur as an extension of granulomatous inflammation. On histopathology, fibrotic changes begin at the periphery of granulomas and extend centrally (Fig 2).21 The dense collagen matrices and fibroblastic foci of IPF are rarely observed in sarcoidosis-associated PF. Paralleling the distribution of granulomatous inflammation, fibrotic changes in sarcoidosis-associated PF are prominent along bronchovascular bundles and septal lines. Whether fibrosis in sarcoidosis also occurs distant from sites of granulomatous inflammation is not known. It is also unknown if persistent granulomatous inflammation is required for progressive fibrosis. Especially late in the disease course, tissue samples may not always reveal granulomas.22 Of interest, in a recent report of the histopathology of explanted lungs from 7 patients with end-stage sarcoidosis, chronic interstitial pneumonitis (CIP), usually an inflammatory feature of early sarcoidosis, was identified in 4.22 This finding challenges the notion that CIP is limited to early disease, and that granulomatous inflammation is the sole active inflammatory process of sarcoidosis-associated PF. Importantly, in those patients with evident CIP, the time course to progression to end-stage disease was considerably shorter than in those without CIP (4.8 vs 23.3 years); in sarcoidosis-associated PF, there may be a subset of patients with a rapidly progressive course marked by distinct immunopathologic characteristics.23 More studies are needed to further define the fibrotic features of sarcoidosis-associated PF, and to establish the roles of granulomatous and nongranulomatous interstitial inflammation in this phenotype.
Fig 2.
Transbronchial biopsy demonstrates multiple well-formed, non-necrotizing granulomas (long arrows) surrounded by an early fibrotic response marked by collagen deposition around fibroblasts (arrow heads). Mild interstitial inflammation is evident in the surrounding alveolar tissue. (Color version of figure is available online.)
IMMUNOPATHOGENESIS
Sarcoidosis is a complex immune disease: the intensity of the inflammatory reaction varies among patients, and inflammation often fluctuates over time in a given patient. In addition, the immunopathogenesis may evolve over the course of disease.24 While much of the immunopathogenesis of sarcoidosis remains poorly understood, granuloma formation is a hallmark event.25 Macrophages and CD4+ T cells are the key effectors of the granulomatous reaction and associated inflammation. In pulmonary sarcoidosis, alveolar macrophages can present antigen, and they release cytokines that contribute to the recruitment, activation, and polarization of CD4+ T cells. The demonstration of the oligoclonal expansion of CD4+ T cells lends strong support to the hypothesis that sarcoidosis is an antigen driven disease.26,27 To date, no antigen has been definitely identified to mediate sarcoidosis, although mycobacterial proteins have emerged as an area of great interest.28 At least initially, CD4+ T cells are polarized to a T helper 1 (Th1) phenotype. A Th1 phenotype drives cell-mediated immune responses, which are marked by the reciprocal recruitment and activation of macrophages. Sarcoid granulomas result from the organized aggregation of these macrophages, which can transform into epithelioid and multinucleated giant cell morphologies. CD4+ T cells, although less abundant than macrophages, are also found within sarcoid granulomas. Other cells, such as CD8+ T cells, B lymphocytes, and fibroblasts form a rim around granulomas.12 It is not clear how or if CD8+ T cells contribute to sarcoid inflammation. B cell activation may occur in sarcoidosis, and in many cases a nonspecific hyperglobulinemia is observed in active disease; however, a definitive role for humoral immunity in the pathogenesis is not established.29
Chemokines and cytokines recruit and activate inflammatory cells, and determine the polarization of CD4+ cells.30 IFN-γ, a Th1 signature cytokine, is thought to be substantially upregulated in early and active sarcoidosis.31 TNF-α is critical in granulomatous inflammation, and TNF-α blockade has been investigated as a therapeutic target in severe disease.32 In addition to IFN-γ and TNF-α, it is likely that a multitude of cytokines is required for the highly coordinated and complex inflammatory response in sarcoidosis. Among others, alterations in IL-1, IL-6, IL-12, IL-15, IL-18, MCP-1, RANTES, GM-CSF, and MIP-1b have been detected in lung samples from patients with pulmonary sarcoidosis.9,26 The correlation and role of cytokines in specific phenotypes of sarcoidosis have not been extensively explored, but are of interest: variations in cytokine patterns are a biologically plausible mechanism of clinical heterogeneity, a remarkable feature of sarcoidosis.
Much of our understanding of the immunopathogenesis of sarcoidosis derives from findings in the acute phase of disease. The immune mechanisms of chronic disease have been less well-studied and are less well-understood. It is not known if chronic disease results from an inability to clear or contain antigen, from fundamental differences in the underlying inflammatory response, or from the failure to downregulate inflammation. There is limited evidence that environmental remediation, as an attempt to alter antigen exposure, is helpful in sarcoidosis. Whether self antigens play a role in sustained sarcoid inflammation is not known. In patients with new-onset sarcoidosis, differences in lung lavage granulocyte counts and TGF-β release have been detected between those who eventually undergo clinical remission and those who develop a chronic disease course.33,34 These suggest that, among sarcoidosis patients, there may be important differences in the initial inflammatory reaction that affect the long-term course. Failure to downregulate inflammation also may be an important contributor to chronic disease. T-regulatory cells (Tregs) are a subset of lymphocytes which downregulate inflammation. An amplified pool of Tregs with a compromised capacity to suppress effector CD4+ T cells has been observed in early, active sarcoidosis.35 The role of Tregs in chronic disease remains unknown, but is of great interest. In addition to Treg function, observed mutations in BTNL2 and ANXA11, whose gene products have anti-inflammatory and immune regulatory properties, support a role of the failure to downregulate and contain inflammation in chronic sarcoidosis.36-39
Fibrosis develops in a subset of patients with chronic active sarcoidosis. However, and particularly when it occurs early in the disease course, fibrosis does not necessarily imply that inflammation is chronically active. Both chronically active disease and residual fibrotic damage result in long-term impairment, and fall under the rubric of chronic sarcoidosis. The distinction between these processes, while not always made explicit, informs the understanding and treatment of sarcoidosis-associated PF. It is not known whether fibrosis is triggered early in sarcoidosis by particularly profibrotic inflammatory events or by an inherent predisposition towards fibrosis in a subset of patients, or whether it evolves as a wound-healing response to uncontrolled, chronic inflammation. Establishing if fibrosis is pre-dominantly an early or late-stage disease event, and the relationship between inflammation and fibrosis are important and will advance our understanding of the underlying immunopathogenesis. In regards to specifically evaluated mechanisms, transforming growth factor beta (TGF-β), the cytokine signature, and macrophages have been explored as candidate abnormalities in sarcoidosis-associated PF. These are explored in turn below.
TGF-β IN SARCOIDOSIS-ASSOCIATED PF
TGF-β is a multifunctional cell signal, with a role in developmental signaling, tissue homeostasis, and inflammation attenuation.40,41 In addition, TGF-β has important wound healing properties, and by extension has been implicated in a variety of fibrotic conditions. As a cytokine and mitogen, TGF-β signals for fibroblast recruitment, activation, proliferation, and transformation to a myofibroblast phenotype, and is implicated in collagen production, epithelial cell apoptosis, and the transformation of epithelial cells to myofibroblasts. In these ways, TGF-β has a potential master regulator role in repairing damaged tissue and also in mediating pathologic fibrosis.42,43
There are 3 isoforms of TGF-β in mammals and, in spite of some functional overlap, each may contribute in different ways to fibrosis.44 In experimental models, increased expression of TGF-β1 leads to a sharp increase in the deposition of extracellular matrix, and this isoform is most strongly implicated in pathologic fibrosis.42 A wound healing effect of TGF-β2 in lung inflammation has been recently reported, although the role of this isoform in fibrotic lung disease is unknown.45 Finally, while it has been suggested that TGF-β3 serves as an antifibrotic signal, the cumulative results are conflicting, and the role of TGF-β3 in fibrosis remains unresolved.9,46,47 There are also several TGF-β receptors, which can utilize different intracellular signaling pathways.44 Therefore, the particular TGF-β response unit of the cell, in addition to modulation from other cytokine signal inputs, influence the effect of TGF-β in a given circumstance.
Despite the role of TGF-β in fibrosis, earlier studies of TGF-β in sarcoidosis demonstrated an association between increased levels in lung samples and the development of spontaneous clinical remission. Given this, TGF-β was thought to have possibly an anti-inflammatory effect early in the course of disease.10,34 There is a dearth of information about TGF-β protein levels in chronic sarcoidosis, and especially in those with sarcoidosis-associated PF. However, TGF-β genes have been evaluated in chronic sarcoidosis. In one study, no association was detected between the TGF-β1 genotype and severe pulmonary sarcoidosis, although importantly the phenotype of PF was not specifically assessed.43,48 In contrast to TGF-β1, TGF-β2 and TGF-β3 genes have been shown to be altered in sarcoidosis-associated PF.9,49 This supports the possibility that intrinsic alterations in TGF-β may be an important predisposing factor in the development of sarcoidosis-associated PF. Correlating TGF-β genotypes with protein levels is essential to better understand the meaning of identified genetic alterations. In addition, studies to correlate TGF-β levels with clinical phenotypes of sarcoidosis will help clarify the role of this cytokine in promoting resolution of disease and/or pathologic fibrosis.
Bone morphogenic proteins (BMPs) belong to the TGF-β superfamily. BMPs stimulate tissue regeneration after injury, but in contrast to TGF-β are not profibrotic. In fact, BMPs, particularly BMP-7 and BMP-4, counter TGF-β-mediated fibrotic processes.50 The balance between TGF-β and BMP signaling may, therefore, be an important determinant of tissue fibrosis after injury. Inhibitors of BMPs have been identified, and may affect this balance. Gremlin, encoded by GREM1, is an important BMP inhibitor.51,52 In sarcoidosis, an association between the GREM1 genotype and the development of sarcoidosis-associated PF has been reported.53 While functional studies are needed to determine the impact of GREM1 polymorphisms on gremlin levels, BMP signaling, and pathologic fibrosis, a strong interest in the role and regulation of BMPs in sarcoidosis-associated PF has been generated.
T HELPER POLARIZATION AND CYTOKINES
While acute sarcoidosis is marked by a Th1 predominate cytokine signature, including the upregulation of IFN-γ, the signatures in chronic disease, and in sarcoidosis-associated PF in particular, are not known. IFN-γ is an antifibrotic signal, and resolving the paradox of fibrosis in IFN-γ mediated sarcoidosis will provide insight into the pathogenesis of sarcoidosis-associated PF.54 It has been suggested that a transition from a Th1 to a Th2 cytokine signature may occur in chronic sarcoidosis, perhaps as a response to persistent inflammation.6,27 Th2 cytokines are often implicated in processes of tissue fibrosis.55,56 As an extension of their role in wound healing, Th2 cytokines can activate and stimulate the proliferation of fibroblasts, and modify the interactions between macrophages and fibroblasts. In fibrotic disease models, the magnitude of the Th2 response, rather than the magnitude of the overall immune response, can be more closely associated with fibrosis severity.54 Establishing the cytokine signature in sarcoidosis-associated PF will, therefore, advance our understanding of the role of cytokines in the development of the fibrotic phenotype.
Several specific Th2 chemokines and cytokines have been identified as potentially particularly relevant in pulmonary fibrosis. Among them, IL-13 and CCL2 have been implicated in the pathogenesis of lung fibrosis models, including in IPF.42,57,58 While both have also been evaluated in sarcoidosis, their role in sarcoidosis-associated PF remains unclear.59-61 IL-13 can stimulate the release of TGF-β, and may act directly via other mechanisms to mediate fibrosis.62,63 In 1 study, IL-13 was found to be elevated in sarcoid lung samples, yet levels were higher in patients without parenchymal disease.64 Importantly, though, stage IV disease was not included in the subgroup analysis. In addition to profibrotic effects, IL-13 has anti-inflammatory properties, including suppression of TNF-α release. It may be that the anti-inflammatory effect of IL-13 has a preventative role for inflammatory parenchymal damage, but that IL-13 could also contribute to fibrotic responses in those who develop sarcoidosis-associated PF. To our knowledge, the role of IL-13 in sarcoidosis-associated PF has not been specifically evaluated. CCL2 promotes fibroblast survival, and also has been demonstrated to be elevated in sarcoid lung samples.65,66 While levels were not associated with CXR stage, stage IV disease was again not represented, limiting the ability to generalize the findings to sarcoidosis-associated PF. Taken together, these findings suggest that in cytokine studies, as in other studies, phenotyping sarcoidosis is important, and pulmonary fibrosis should be specifically evaluated. It remains to be determined how different or more complex cell signaling is in sarcoidosis-associated PF compared with IPF.
MACROPHAGES IN SARCOIDOSIS-ASSOCIATED PF
Macrophages are the architectural basis of sarcoid granulomas, and in many ways play a central role in sarcoid inflammation. Macrophages are also a target of interest in pathologic fibrosis, and in sarcoidosis the altered polarization of macrophages may be important in the transition from an inflammatory to fibrotic state.67,68 ATh1 cytokine milieu leads to classical activation of macrophages and the development of a proinflammatory M1 polarization. Alternative activation occurs in the setting of a Th2 cytokine milieu, and favors development of a wound healing M2 polarization. As pathological fibrosis suggests aberrant or inappropriate wound healing, M2 macrophages are implicated in the pathogenesis of fibrosis. Indeed, a M2 polarization of alveolar macrophages has been demonstrated in fibrotic lung diseases, including in sarcoidosis-associated PF.69
M2 polarized macrophages may contribute to fibrosis in various ways. Perhaps most significantly, they are potent producers of TGF-β.70 In addition to TGF-β, they also produce other cell signals which stimulate fibroblasts. Among these, CCL18 has been directly implicated in fibrosis.68 While the receptor for CCL18 is unknown, and the downstream signaling events have yet to be fully elucidated, CCL18 production is associated with fibroblast stimulation and augmented collagen production.68,71 In sarcoidosis, increased CCL18 was associated with sarcoidosis-associated PF but not with other phenotypes of pulmonary disease.69,71 While the role of CCL18 merits further study, the finding thus far of a phenotype-restricted increase in CCL18 supports a M2 polarization of macrophages in sarcoidosis-associated PF, and suggests a pathogenic role of these macrophages in the fibrotic response.
Arginine utilization is another biologically plausible mechanism by which M2 polarized macrophages can contribute to fibrosis.72 M1 polarized macrophages preferentially produce inducible nitric oxide synthase, which metabolizes arginine to nitric oxide. In addition to being an important modifier of vascular function, nitric oxide is proinflammatory.73,74 In contrast, M2 polarized macrophages preferentially express Arg1, which results in increased arginase. Arginase metabolizes arginine to ornithine, a precursor to the collagen substrate proline.54,64,75 In this way, through the selective utilization of arginine pathways, the polarization of macrophages influences their capacity to either promote inflammation or to produce the building blocks of fibrosis. In animal models, a Th2 cytokine signature, thought to promote M2 polarization, has been shown to correlate with expression of Arg1 and with the development of fibrosis.76 Further studies of macrophage polarization and arginine pathways in human fibrosis samples are indicated. However, the findings of a M2 polarization in fibrotic lung conditions, including sarcoidosis-associated PF, raise the possibility that elaboration of arginase has a pathogenic role in the fibrotic response.
M1 and M2 polarizations are not necessarily terminally differentiated states, and activated macrophages may modify their phenotype in response to changes in the cytokine milieu.77,78 Furthermore, the dichotomy of M1 and M2 may be too simplistic; likely, an array of macrophage phenotypes exists, with graduated tendencies towards inflammation or wound healing and fibrosis. Nevertheless, further study of alveolar macrophage polarization and function in sarcoidosis-associated PF should be revealing, as it has been in the study of other fibrotic diseases. In addition to the finding of a M2 polarization of alveolar macrophages in sarcoidosis-associated PF, a M2 polarization has also been demonstrated in fibrotic muscular sarcoidosis.79 We recognize that not all studies have demonstrated an altered macrophage polarization in sarcoidosis, particularly when the phenotype of pulmonary fibrosis is excluded from subgroup analyses.80 As the clinical heterogeneity of sarcoidosis is remarkable, studying patients collectively may obscure the detection of signals important for specific phenotypes, such as sarcoidosis-associated PF. As this work continues, it remains to be determined if M2 macrophages in sarcoidosis-associated PF enhance fibrotic events, or if they are the incidental result of other primary processes which drive fibrosis.
GENE EXPRESSION IN SARCOIDOSIS-ASSOCIATED PF
In a landmark study of expression profiling in sarcoidosis, the profiles in lung samples from patients with active yet non-fibrotic disease, and in samples from those with active progressive disease and mild pulmonary fibrosis were distinct.16 Much of the difference in gene expression between these groups could be attributed to an upregulation of inflammation genes in the fibrotic group, with only minimal activation of these genes in the nonfibrotic group. Importantly, disease activity was higher in the cohort with fibrosis; controlling for disease activity in future studies will be important to more definitively implicate active inflammation in fibrosis. In addition, fibrosis was mild as subjects had essentially normal pulmonary function; it remains to be determined if these subjects develop extensive, clinically relevant fibrosis. However, the finding that an inflammatory signature is associated with the development of sarcoidosis-associated PF, at least in active disease, has important therapeutic implications.
In another study of gene expression in sarcoidosis, profiles in blood and lung samples were compared.81 Using lung samples and the phenotype categories from the Lockstone study, expression profiles in phenotyped lung samples were compared with nonphenotyped sarcoidosis blood samples from different patients. In those with active progressive disease and mild fibrosis, there was an overlap in the expression profiles of lung and blood compartments. This suggests that the circulating immune system holds promise as an investigative site in sarcoidosis-associated PF. In this same study, in a cohort of patients with nonphenotyped sarcoidosis, expression profiles in lung and blood samples from the same patients were directly compared. In this combined phenotype analysis, therewas a remarkable correlation between lung and blood expression profiles. This finding again challenges the notion of a strict compartmentalization of inflammation in sarcoidosis. Correlating expression profiles between lung and blood samples from the same patients with extensive fibrosis will further establish the utility of circulating gene expression in sarcoidosis-associated PF.
PULMONARY FIBROSIS IN SARCOIDOSIS AND IPF
Idiopathic pulmonary fibrosis is the prototypical fibrotic lung disease. Findings in studies of IPF have been a catalyst and a reference point for studies of other fibrotic lung conditions, including sarcoidosis-associated PF. However, there are important differences in the clinical and immunologic features that suggest that the disease model in IPF may not be entirely generalizable to sarcoidosis-associated PF.
The patient demographics, radiographic features, and long-term outcomes of IPF and sarcoidosis-associated PF clinically distinguish these diseases. While IPF is more common in elderly, Caucasian men, most patients with sarcoidosis-associated PF in the United States are middle aged, and African Americans and females are disproportionally affected.82-84 In contrast to the peripheral and lower lobe distribution of honeycombing fibrosis in IPF, sarcoidosis-associated PF is marked by upper lobe involvement and a predominance of nonhoneycombing fibrosis. When chronic obstructive pulmonary disease is not a coexisting disease, obstruction onpulmonary function testing is rare in IPF. In contrast, obstruction is relatively common in patients with sarcoidosis-associated PF. Finally, the clinical courses are distinct for these 2 conditions. Idiopathic pulmonary fibrosis typically progresses quickly, with most patients succumbing to disease within 5 years of diagnosis, whereas sarcoidosis-associated PF is often a chronic disease with a lower mortality rate.17
Animal models, while lacking in sarcoidosis, have been invaluable in the study of IPF. Therefore, the mechanisms of IPF have been more extensively explored than those of sarcoidosis-associated PF. Awidely accepted hypothesis of IPF centers around repetitive epithelial damage with abnormal wound healing responses.85,86 Whether epithelial damage results from inflammatory or noninflammatory microinjuries remains an active area of debate.87 Abnormal or dysregulated wound healing events in IPF include the recruitment of mesenchymal cells, fibroblasts, and macrophages, resulting in matrix deposition and parenchymal damage. In contrast, it is not clear that epithelial damage with abnormal wound healing contributes to the pathogenesis of sarcoidosis-associated PF. Cytokines, growth factors, and proteases are all thought to contribute in important ways to fibrosis and tissue damage in IPF; evaluating these factors in sarcoidosis-associated PF will advance our understanding of their role, if any, in this disease as well. Additionally, altered prostaglandin homeostasis has been implicated in IPF.88,89 Prostaglandins can have antifibrotic effects, and prostaglandin (PG)E2 in particular has been identified as an important factor in mitigating fibrotic damage.90 Levels of PGE2 have been shown to be substantially diminished in IPF, and the role of PGE2 in sarcoidosis-associated PF is of growing interest.91 Prostaglandin-endoperoxide synthase 2 is a key regulatory enzyme in the synthesis of PGE2. An altered polymorphism in the promoter region of this gene was identified in a cohort of patients with progressive sarcoidosis, which included patients with fibrotic disease.92 This identifies an important area for further study. Finally, while the levels of Tregs in both blood and lung samples have been shown to be decreased in IPF, the role of Tregs in sarcoidosis-associated PF is not known.93
The number of studies directly comparing IPF and sarcoidosis-associated PF is limited. However, gene expression profiles in these diseases were recently compared.16 Remarkably, there was no enrichment of the IPF gene set in those with mild sarcoidosis-associated PF. While there are likely shared features of disease, the results of this study strongly suggest that there are also important differences between the mechanisms of lung fibrosis in sarcoidosis-associated PF and IPF. Ongoing work to define and compare the genetic signature in these diseases should further delineate the differences and similarities of their fibrotic pathways.
CONCLUSIONS
Pulmonary fibrosis is an important yet poorly defined and understudied phenotype of sarcoidosis. Sarcoidosis-associated PF often leads to substantial morbidity and can be fatal. However, the clinical features, long-term complications, and fibrosis patterns can vary tremendously among patients. The extent to which the underlying pathophysiology is also variable among patients with this phenotype is not known. Updated diagnostic criteria are needed. Historically, sarcoidosis-associated PF has been diagnosed by Scadding stage IV findings on chest x-ray. However, HRCT imaging, which is now commonly employed, is more sensitive for fibrotic changes. It will be important to qualify ways of distinguishing small, incidental areas of fibrosis from extensive fibrosis. The latter is more likely to be physiologically and, therefore, clinically relevant. Systemic corticosteroids are associated with substantial side effects and have not been demonstrated to be beneficial in sarcoidosis-associated PF. We suggest that treatment in sarcoidosis-associated PF be driven by clinical suggestion of active inflammation. Sarcoidosis-associated PF is, by nature of the irreversibility of fibrotic damage, a chronic condition. Distinguishing between active granulomatous activity and residual fibrosis is an important, albeit potentially challenging part of the clinical care of patients with sarcoidosis-associated PF.
Recent results from studies of gene expression suggest that sarcoidosis-associated PF develops in the setting of inflammation. However, it is not known how inflammation in fibrotic sarcoidosis compares with inflammation in nonfibrotic sarcoidosis. In this regard, cytokine patterns are of particular interest. We have highlighted recent findings of alterations in the genes for TGF-β and GREM1 in sarcoidosis-associated PF. Follow-up studies to correlate TGF-β gentoypes with TGF-β protein levels and clinical phenotypes will be informative. Th2 cytokines, which influence TGF-β production and macrophage polarization, may also be important cell signals in sarcoidosis-associated PF. Defining the T helper cytokine signature will therefore advance our understanding of the mechanisms of fibrotic sarcoidosis. The M2 polarization of macrophages is thought to be important in lung fibrosis, and accumulating data suggest that macrophages may be skewed towards this polarization in sarcoidosis-associated PF. This, in our opinion, is a particularly compelling avenue for further investigation.
Establishing the natural history of sarcoidosis-associated PF is important. It remains to be determined if sarcoidosis-associated PF is a predisposed phenotype that begins early in the disease course, or whether it develops as a response to unrestrained or unremitting inflammation. It also remains to be determined if sarcoidosis-associated PF, once initiated, can progress independently from active granulomatous inflammation. Finally, the extent to which the immunopathologies of other fibrotic lung diseases overlap with that of sarcoidosis-associated PF is not known. However, the clinical and immunologic features of IPF and sarcoidosis-associated PF strongly suggest that at least some distinct mechanisms underlie these diseases.
Abbreviations
- ANXA11
annexin A11
- Arg1
arginase 1
- BTNL2
butyrophilin-like protein 2
- BMPs
bone morphogenic proteins
- CIP
chronic interstitial pneumonitis
- CXR
chest x-ray
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- GREM1
gremlin 1
- HRCT
high resolution computed tomography
- IFN-γ
interferon gamma
- IPF
idiopathic pulmonary fibrosis
- M1
classically activated macrophage
- M2 macrophage
alternatively activated macrophage
- MCP-1
monocyte chemotactic protein-1
- MIP-1b
macrophage-inflammatory protein-1b
- PF
pulmonary fibrosis
- PGE2
prostaglandin E2
- RANTES
regulated on activation, normal T cell expressed and secreted
- TGF-β
transforming growth factor-b
- Th1
T helper 1
- Th2
T helper 2
- Tregs
T regulatory cells
- TNF-α
tumor necrosis factor-α
Footnotes
Conflict of interests: None.
REFERENCES
- 1.Prasse A, Katic C, Germann M, Buchwald A, Zissel G, Muller-Quernheim J. Phenotyping sarcoidosis from a pulmonary perspective. Am J Respir Crit Care Med. 2008;177:330–6. doi: 10.1164/rccm.200705-742OC. [DOI] [PubMed] [Google Scholar]
- 2.Iannuzzi MC, Fontana JR. Sarcoidosis: clinical presentation, immunopathogenesis, and therapeutics. JAMA. 2011;305:391–9. doi: 10.1001/jama.2011.10. [DOI] [PubMed] [Google Scholar]
- 3.Baughman RP, Winget DB, Bowen EH, Lower EE. Predicting respiratory failure in sarcoidosis patients. Sarcoidosis Vasc Diffuse Lung Dis. 1997;14:154–8. [PubMed] [Google Scholar]
- 4.Hennebicque AS, Nunes H, Brillet PY, Moulahi H, Valeyre D, Brauner MW. CT findings in severe thoracic sarcoidosis. Eur Radiol. 2005;15:23–30. doi: 10.1007/s00330-004-2480-4. [DOI] [PubMed] [Google Scholar]
- 5.Gideon NM, Mannino DM. Sarcoidosis mortality in the United States 1979-1991: an analysis of multiple-cause mortality data. Am J Med. 1996;100:423–7. doi: 10.1016/S0002-9343(97)89518-6. [DOI] [PubMed] [Google Scholar]
- 6.Teirstein AT, Morgenthau AS. “End-stage” pulmonary fibrosis in sarcoidosis. Mt Sinai J Med. 2009;76:30–6. doi: 10.1002/msj.20090. [DOI] [PubMed] [Google Scholar]
- 7.Nardi A, Brillet PY, Letoumelin P, et al. Stage IV sarcoidosis: comparison of survival with the general population and causes of death. Eur Respir J. 2011;38:1368–73. doi: 10.1183/09031936.00187410. [DOI] [PubMed] [Google Scholar]
- 8.Swigris JJ, Olson AL, Huie TJ, et al. Sarcoidosis-related mortality in the United States from 1988 to 2007. Am J Respir Crit Care Med. 2011;183:1524–30. doi: 10.1164/rccm.201010-1679OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lynch JP, III, Ma YL, Koss MN, White ES. Pulmonary sarcoidosis. Semin Respir Crit Care Med. 2007;28:53–74. doi: 10.1055/s-2007-970333. [DOI] [PubMed] [Google Scholar]
- 10.Pabst S, Franken T, Schonau J, Stier S, Nickenig G, Meyer R, et al. Transforming growth factor-β gene polymorphisms in different phenotypes of sarcoidosis. Eur Respir J. 2011;38:169–75. doi: 10.1183/09031936.00120410. [DOI] [PubMed] [Google Scholar]
- 11.Abehsera M, Valeyre D, Grenier P, Jaillet H, Battesti JP, Brauner MW. Sarcoidosis with pulmonary fibrosis: CT patterns and correlation with pulmonary function. AJR Am J Roentgenol. 2000;174:1751–7. doi: 10.2214/ajr.174.6.1741751. [DOI] [PubMed] [Google Scholar]
- 12.Ma Y, Gal A, Koss MN. The pathology of pulmonary sarcoidosis: update. Semin Diagn Pathol. 2007;24:150–61. doi: 10.1053/j.semdp.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 13.Lavergne F, Clerici C, Sadoun D, Brauner M, Battesti JP, Valeyre D. Airway obstruction in bronchial sarcoidosis: outcome with treatment. Chest. 1999;116:1194–9. doi: 10.1378/chest.116.5.1194. [DOI] [PubMed] [Google Scholar]
- 14.Arcasoy SM, Christie JD, Pochettino A, et al. Characteristics and outcomes of patients with sarcoidosis listed for lung transplantation. Chest. 2001;120:873–80. doi: 10.1378/chest.120.3.873. [DOI] [PubMed] [Google Scholar]
- 15.Nunes H, Humbert M, Capron F, et al. Pulmonary hypertension associated with sarcoidosis: mechanisms, haemodynamics and prognosis. Thorax. 2006;61:68–74. doi: 10.1136/thx.2005.042838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lockstone HE, Sanderson S, Kulakova N, et al. Gene set analysis of lung samples provides insight into pathogenesis of progressive, fibrotic pulmonary sarcoidosis. Am J Respir Crit Care Med. 2010;181:1367–75. doi: 10.1164/rccm.200912-1855OC. [DOI] [PubMed] [Google Scholar]
- 17.King TE, Jr, Tooze JA, Schwarz MI, Brown KR, Cherniack RM. Predictingsurvival inidiopathic pulmonary fibrosis: scoringsystem and survival model. Am J Respir Crit Care Med. 2001;164:1171–81. doi: 10.1164/ajrccm.164.7.2003140. [DOI] [PubMed] [Google Scholar]
- 18.Judson MA. An approach to the treatment of pulmonary sarcoidosis with corticosteroids: the six phases of treatment. Chest. 1999;115:1158–65. doi: 10.1378/chest.115.4.1158. [DOI] [PubMed] [Google Scholar]
- 19.Scadding JG. A “burnt-out” case of sarcoidosis. Postgrad Med J. 1968;44:105–8. doi: 10.1136/pgmj.44.507.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Remy-Jardin M, Giraud F, Remy J, Wattinne L, Wallaert B, Duhamel A. Pulmonary sarcoidosis: role of CT in the evaluation of disease activity and functional impairment and in prognosis assessment. Radiology. 1994;191:675–80. doi: 10.1148/radiology.191.3.8184045. [DOI] [PubMed] [Google Scholar]
- 21.Criado E, Sanchez M, Ramirez J, et al. Pulmonary sarcoidosis: typical and atypical manifestations at high-resolution CT with pathologic correlation. Radiographics. 2010;30:1567–86. doi: 10.1148/rg.306105512. [DOI] [PubMed] [Google Scholar]
- 22.Shigemitsu H, Oblad JM, Sharma OP, Koss MN. Chronic interstitial pneumonitis in end-stage sarcoidosis. Eur Respir J. 2010;35:695–7. doi: 10.1183/09031936.00150609. [DOI] [PubMed] [Google Scholar]
- 23.Kim DS, Collard HR, King TE., Jr. Classification and natural history of the idiopathic interstitial pneumonias. Proc Am Thorac Soc. 2006;3:285–92. doi: 10.1513/pats.200601-005TK. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mollers M, Aries SP, Dromann D, Mascher B, Braun J, Dalhoff K. Intracellular cytokine repertoire in different T cell subsets from patients with sarcoidosis. Thorax. 2001;56:487–93. doi: 10.1136/thorax.56.6.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Newman LS, Rose CS, Maier LA. Sarcoidosis. N Engl J Med. 1997;336:1224–34. doi: 10.1056/NEJM199704243361706. [DOI] [PubMed] [Google Scholar]
- 26.Moller DR. Cells and cytokines involved in the pathogenesis of sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 1999;16:24–31. [PubMed] [Google Scholar]
- 27.Moller DR. Pulmonary fibrosis of sarcoidosis. New approaches, old ideas. Am J Respir Cell Mol Biol. 2003;29:S37–41. [PubMed] [Google Scholar]
- 28.Moller DR. Potential etiologic agents in sarcoidosis. Proc Am Thorac Soc. 2007;4:465–8. doi: 10.1513/pats.200608-155MS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hunninghake GW, Crystal RG. Mechanisms of hypergammaglobulinemia in pulmonary sarcoidosis. Site ofincreased antibodyproduction and role of T lymphocytes. J Clin Invest. 1981;67:86–92. doi: 10.1172/JCI110036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wahlstrom J, Katchar K, Wigzell H, Olerup O, Eklund A, Grunewald J. Analysis of intracellular cytokines in CD4+ and CD8+ lung and blood T cells in sarcoidosis. Am J Respir Crit Care Med. 2001;163:115–21. doi: 10.1164/ajrccm.163.1.9906071. [DOI] [PubMed] [Google Scholar]
- 31.Muller-Quernheim J. Sarcoidosis: immunopathogenetic concepts and their clinical application. Eur Respir J. 1998;12:716–38. doi: 10.1183/09031936.98.12030716. [DOI] [PubMed] [Google Scholar]
- 32.Antoniu SA. Targeting the TNF-α pathway in sarcoidosis. Expert Opin Ther Targets. 2010;14:21–9. doi: 10.1517/14728220903449244. [DOI] [PubMed] [Google Scholar]
- 33.Drent M, Jacobs JA, de Vries J, Lamers RJ, Liem IH, Wouters EF. Does the cellular bronchoalveolar lavage fluid profile reflect the severity of sarcoidosis? Eur Respir J. 1999;13:1338–44. doi: 10.1183/09031936.99.13613459. [DOI] [PubMed] [Google Scholar]
- 34.Zissel G, Homolka J, Schlaak J, Schlaak M, Muller-Quernheim J. Anti-inflammatorycytokine release byalveolar macrophagesinpulmonary sarcoidosis. Am J Respir Crit Care Med. 1996;154:713–9. doi: 10.1164/ajrccm.154.3.8810610. [DOI] [PubMed] [Google Scholar]
- 35.Miyara M, Amoura Z, Parizot C, et al. The immune paradox of sarcoidosis and regulatory T cells. J Exp Med. 2006;203:359–70. doi: 10.1084/jem.20050648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hofmann S, Franke A, Fischer A, et al. Genome-wide association study identifies ANXA11 as a new susceptibility locus for sarcoidosis. Nat Genet. 2008;40:1103–6. doi: 10.1038/ng.198. [DOI] [PubMed] [Google Scholar]
- 37.Valentonyte R, Hampe J, Huse K, et al. Sarcoidosis is associated with a truncating splice site mutation in BTNL2. Nat Genet. 2005;37:357–64. doi: 10.1038/ng1519. [DOI] [PubMed] [Google Scholar]
- 38.Hayes MJ, Longbottom RE, Evans MA, Moss SE. Annexinopathies. Subcell Biochem. 2007;45:1–28. doi: 10.1007/978-1-4020-6191-2_1. [DOI] [PubMed] [Google Scholar]
- 39.Nguyen T, Liu XK, Zhang Y, Dong C. BTNL2, a butyrophilin-like molecule that functions to inhibit T cell activation. J Immunol. 2006;176:7354–60. doi: 10.4049/jimmunol.176.12.7354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wahl SM, Swisher J, McCartney-Francis N, Chen W. TGF-β: the perpetrator of immune suppression by regulatory T cells and suicidal T cells. J Leukoc Biol. 2004;76:15–24. doi: 10.1189/jlb.1103539. [DOI] [PubMed] [Google Scholar]
- 41.Goodwin A, Jenkins G. Role of integrin-mediated TGFβ activation in the pathogenesis of pulmonary fibrosis. Biochem Soc Trans. 2009;37:849–54. doi: 10.1042/BST0370849. [DOI] [PubMed] [Google Scholar]
- 42.Gharaee-Kermani M, Hu B, Phan SH, Gyetko MR. Recent advances in molecular targets and treatment of idiopathic pulmonary fibrosis: focus on TGFβ signaling and the myofibroblast. Curr Med Chem. 2009;16:1400–7. doi: 10.2174/092986709787846497. [DOI] [PubMed] [Google Scholar]
- 43.Xaubet A, Marin-Arguedas A, Lario S, et al. Transforming growth factor-β1 gene polymorphisms are associated with disease progression in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2003;168:431–5. doi: 10.1164/rccm.200210-1165OC. [DOI] [PubMed] [Google Scholar]
- 44.Border WA, Noble NA. Transforming growth factor b in tissue fibrosis. N Engl J Med. 1994;331:1286–92. doi: 10.1056/NEJM199411103311907. [DOI] [PubMed] [Google Scholar]
- 45.Ito J, Harada N, Nagashima O, et al. Wound-induced TGF-β1 and TGF-β2 enhance airway epithelial repair via HB-EGF and TGF-α. Biochem Biophys Res Commun. 2011;412:109–14. doi: 10.1016/j.bbrc.2011.07.054. [DOI] [PubMed] [Google Scholar]
- 46.Widgerow AD. Cellular/extracellular matrix cross-talk in scar evolution and control. Wound Repair Regen. 2011;19:117–33. doi: 10.1111/j.1524-475X.2010.00662.x. [DOI] [PubMed] [Google Scholar]
- 47.Coker RK, Laurent GJ, Jeffery PK, du Bois RM, Black CM, McAnulty RJ. Localisation of transforming growth factor β1 and β3 mRNA transcripts in normal and fibrotic human lung. Thorax. 2001;56:549–56. doi: 10.1136/thorax.56.7.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Murakozy G, Gaede KI, Zissel G, Schlaak M, Muller-Quernheim J. Analysis of gene polymorphisms in interleukin-10 and transforming growth factor-β 1 in sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2001;18:165–9. [PubMed] [Google Scholar]
- 49.Kruit A, Grutters JC, Ruven HJ, et al. Transforming growth factor-β gene polymorphisms in sarcoidosis patients with and without fibrosis. Chest. 2006;129:1584–91. doi: 10.1378/chest.129.6.1584. [DOI] [PubMed] [Google Scholar]
- 50.Yoshida T, Ohnuma A, Horiuchi H, Harada T. Pulmonary fibrosis in response to environmental cues and molecular targets involved in its pathogenesis. J Toxicol Pathol. 2011;24:9–24. doi: 10.1293/tox.24.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Farkas L, Farkas D, Gauldie J, Warburton D, Shi W, Kolb M. Transient overexpression of Gremlin results in epithelial activation and reversible fibrosis in rat lungs. Am J Respir Cell Mol Biol. 2011;44:870–8. doi: 10.1165/rcmb.2010-0070OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Myllarniemi M, Lindholm P, Ryynanen MJ, et al. Gremlin-mediated decrease in bone morphogenetic protein signaling promotes pulmonary fibrosis. Am J Respir Crit Care Med. 2008;177:321–9. doi: 10.1164/rccm.200706-945OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Heron M, van Moorsel CH, Grutters JC, et al. Genetic variation in GREM1 is a risk factor for fibrosis in pulmonary sarcoidosis. Tissue Antigens. 2011;77:112–7. doi: 10.1111/j.1399-0039.2010.01590.x. [DOI] [PubMed] [Google Scholar]
- 54.Wynn TA. Fibrotic disease and the T(H)1/T(H)2 paradigm. Nat Rev Immunol. 2004;4:583–94. doi: 10.1038/nri1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Barbarin V, Xing Z, Delos M, Lison D, Huaux F. Pulmonary over-expression of IL-10 augments lung fibrosis and Th2 responses induced by silica particles. Am J Physiol Lung Cell Mol Physiol. 2005;288:L841–8. doi: 10.1152/ajplung.00329.2004. [DOI] [PubMed] [Google Scholar]
- 56.Wynn TA. Fibrotic disease and the T(H)1/T(H)2 paradigm. Nat Rev Immunol. 2004;4:583–94. doi: 10.1038/nri1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wen FQ, Kohyama T, Liu X, et al. Interleukin-4- and interleukin-13-enhanced transforming growth factor-β2 production in cultured human bronchial epithelial cells is attenuated by interferon-γ. Am J Respir Cell Mol Biol. 2002;26:484–90. doi: 10.1165/ajrcmb.26.4.4784. [DOI] [PubMed] [Google Scholar]
- 58.Gharaee-Kermani M, Denholm EM, Phan SH. Costimulation of fibroblast collagen and transforming growth factor β1 gene expression by monocyte chemoattractant protein-1 via specific receptors. J Biol Chem. 1996;271:17779–84. doi: 10.1074/jbc.271.30.17779. [DOI] [PubMed] [Google Scholar]
- 59.Park SW, Ahn MH, Jang HK, et al. Interleukin-13 and its receptors in idiopathic interstitial pneumonia: clinical implications for lung function. J Korean Med Sci. 2009;24:614–20. doi: 10.3346/jkms.2009.24.4.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rose CE, Jr, Sung SS, Fu SM. Significant involvement of CCL2 (MCP-1) in inflammatory disorders of the lung. Microcirculation. 2003;10:273–8. doi: 10.1038/sj.mn.7800193. [DOI] [PubMed] [Google Scholar]
- 61.Kunkel SL, Chensue SW, Lukacs N, Hogaboam C. Cytokine phenotypes serve as a paradigms for experimental immune-mediated lung diseasesand remodeling. Am J Respir Cell Mol Biol. 2003;29:S63–6. [PubMed] [Google Scholar]
- 62.MacDonald TT. Decoy receptor springs to life and eases fibrosis. Nat Med. 2006;12:13–4. doi: 10.1038/nm0106-13. [DOI] [PubMed] [Google Scholar]
- 63.Kaviratne M, Hesse M, Leusink M, et al. IL-13 activates a mechanism of tissue fibrosis that is completely TGF-β independent. J Immunol. 2004;173:4020–9. doi: 10.4049/jimmunol.173.6.4020. [DOI] [PubMed] [Google Scholar]
- 64.Hauber HP, Gholami D, Meyer A, Pforte A. Increased interleukin-13 expression in patients with sarcoidosis. Thorax. 2003;58:519–24. doi: 10.1136/thorax.58.6.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Palchevskiy V, Hashemi N, Weigt SS, et al. Immune response CC chemokines CCL2 and CCL5 are associated with pulmonary sarcoidosis. Fibrogenesis Tissue Repair. 2011;4:10. doi: 10.1186/1755-1536-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu X, Das AM, Seideman J, et al. The CC chemokine ligand 2 (CCL2) mediates fibroblast survival through IL-6. Am J Respir Cell Mol Biol. 2007;37:121–8. doi: 10.1165/rcmb.2005-0253OC. [DOI] [PubMed] [Google Scholar]
- 67.Reynolds HY. Lung inflammation and fibrosis: an alveolar macrophage-centered perspective from the 1970s to 1980s. Am J Respir Crit Care Med. 2005;171:98–102. doi: 10.1164/rccm.200406-788PP. [DOI] [PubMed] [Google Scholar]
- 68.Prasse A, Pechkovsky DV, Toews GB, et al. A vicious circle of alveolar macrophages and fibroblasts perpetuates pulmonary fibrosis via CCL18. Am J Respir Crit Care Med. 2006;173:781–92. doi: 10.1164/rccm.200509-1518OC. [DOI] [PubMed] [Google Scholar]
- 69.Pechkovsky DV, Prasse A, Kollert F, et al. Alternatively activated alveolar macrophages in pulmonary fibrosis-mediator production and intracellular signal transduction. Clin Immunol. 2010;137:89–101. doi: 10.1016/j.clim.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 70.Liu G, Ma H, Qiu L, et al. Phenotypic and functional switch of macrophages induced by regulatory CD4+CD251 T cells in mice. Immunol Cell Biol. 2011;89:130–42. doi: 10.1038/icb.2010.70. [DOI] [PubMed] [Google Scholar]
- 71.Luzina IG, Tsymbalyuk N, Choi J, et al. CCL18-stimulated upregulation of collagen production in lung fibroblasts requires Sp1 signaling and basal Smad3 activity. J Cell Physiol. 2006;206:221–8. doi: 10.1002/jcp.20452. [DOI] [PubMed] [Google Scholar]
- 72.Mosser DM. The many faces of macrophage activation. J Leukoc Biol. 2003;73:209–12. doi: 10.1189/jlb.0602325. [DOI] [PubMed] [Google Scholar]
- 73.Miller MJ, Grisham MB. Nitric oxide as a mediator of inflammation?-You had better believe it. Mediators Inflamm. 1995;4:387–96. doi: 10.1155/S0962935195000639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol. 2009;27:451–83. doi: 10.1146/annurev.immunol.021908.132532. [DOI] [PubMed] [Google Scholar]
- 75.Benson RC, Hardy KA, Morris CR. Arginase and arginine dysregulation in asthma. J Allergy (Cairo) 2011;2011:736319. doi: 10.1155/2011/736319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Munder M, Eichmann K, Modolell M. Alternative metabolic states in murine macrophages reflected by the nitric oxide synthase/arginase balance: competitive regulation by CD4+ T cells correlates with Th1/Th2 phenotype. J Immunol. 1998;160:5347–54. [PubMed] [Google Scholar]
- 77.Porcheray F, Viaud S, Rimaniol AC, et al. Macrophage activation switching: an asset for the resolution of inflammation. Clin Exp Immunol. 2005;142:481–9. doi: 10.1111/j.1365-2249.2005.02934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stout RD, Suttles J. Functional plasticity of macrophages: reversible adaptation to changing microenvironments. J Leukoc Biol. 2004;76:509–13. doi: 10.1189/jlb.0504272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Prokop S, Heppner FL, Goebel HH, Stenzel W. M2 polarized macrophages and giant cells contribute to myofibrosis in neuro-muscular sarcoidosis. Am J Pathol. 2011;178:1279–86. doi: 10.1016/j.ajpath.2010.11.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wiken M, Idali F, Al Hayja MA, Grunewald J, Eklund A, Wahlstrom J. No evidence of altered alveolar macrophage polarization, but reduced expression of TLR2, in bronchoalveolar lavage cells in sarcoidosis. Respir Res. 2010;11:121. doi: 10.1186/1465-9921-11-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Koth LL, Solberg OD, Peng JC, Bhakta NR, Nguyen CP, Woodruff PG. Sarcoidosis blood transcriptome reflects lung inflammation and overlaps with tuberculosis. Am J Respir Crit Care Med. 2011;184:1153–63. doi: 10.1164/rccm.201106-1143OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rybicki BA, Maliarik MJ, Major M, Popovich J, Jr, Iannuzzi MC. Epidemiology, demographics, and genetics of sarcoidosis. Semin Respir Infect. 1998;13:166–73. [PubMed] [Google Scholar]
- 83.American Thoracic Society Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. American Thoracic Society (ATS), and the European Respiratory Society (ERS) Am J Respir Crit Care Med. 2000;161:646–64. doi: 10.1164/ajrccm.161.2.ats3-00. [DOI] [PubMed] [Google Scholar]
- 84.Rybicki BA, Major M, Popovich J, Jr, Maliarik MJ, Iannuzzi MC. Racial differences in sarcoidosis incidence: a 5-year study in a health maintenance organization. Am J Epidemiol. 1997;145:234–41. doi: 10.1093/oxfordjournals.aje.a009096. [DOI] [PubMed] [Google Scholar]
- 85.Selman M, King TE, Pardo A. Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann Intern Med. 2001;134:136–51. doi: 10.7326/0003-4819-134-2-200101160-00015. [DOI] [PubMed] [Google Scholar]
- 86.Wynn TA. Integrating mechanisms of pulmonary fibrosis. J Exp Med. 2011;208:1339–50. doi: 10.1084/jem.20110551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bringardner BD, Baran CP, Eubank TD, Marsh CB. The role of inflammation in the pathogenesis of idiopathic pulmonary fibrosis. Antioxid Redox Signal. 2008;10:287–301. doi: 10.1089/ars.2007.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bonner JC, Rice AB, Ingram JL, et al. Susceptibility of cyclooxygenase-2-deficient mice to pulmonary fibrogenesis. Am J Pathol. 2002;161:459–70. doi: 10.1016/S0002-9440(10)64202-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wilborn J, Crofford LJ, Burdick MD, Kunkel SL, Strieter RM, Peters-Golden M. Cultured lung fibroblasts isolated from patients with idiopathic pulmonary fibrosis have a diminished capacity to synthesize prostaglandin E2 and to express cyclooxygenase-2. J Clin Invest. 1995;95:1861–8. doi: 10.1172/JCI117866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bozyk PD, Moore BB. Prostaglandin E2 and the pathogenesis of pulmonary fibrosis. Am J Respir Cell Mol Biol. 2011;45:445–52. doi: 10.1165/rcmb.2011-0025RT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Borok Z, Gillissen A, Buhl R, et al. Augmentation of functional prostaglandin E levels on the respiratory epithelial surface by aerosol administration of prostaglandin E. Am Rev Respir Dis. 1991;144:1080–4. doi: 10.1164/ajrccm/144.5.1080. [DOI] [PubMed] [Google Scholar]
- 92.Hill MR, Papafili A, Booth H, et al. Functional prostaglandin-endoperoxide synthase 2 polymorphism predicts poor outcome in sarcoidosis. Am J Respir Crit Care Med. 2006;174:915–22. doi: 10.1164/rccm.200512-1839OC. [DOI] [PubMed] [Google Scholar]
- 93.Kotsianidis I, Nakou E, Bouchliou I, et al. Global impairment of CD4+CD25+FOXP3+ regulatory T cells in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2009;179:1121–30. doi: 10.1164/rccm.200812-1936OC. [DOI] [PubMed] [Google Scholar]