Abstract
Little is known about the association between iodine and human milk composition. In this study, we investigated the association between iodine and different markers of oxidative stress and obesity-related hormones in human breast milk. This work is composed of two cross-sectional studies (in lactating women and in the general population), one prospective and one in vitro. In the cross-sectional study in lactating women, the breast milk iodine correlated negatively with superoxide dismutase (SOD), catalase, and glutathione peroxidase (GSH-Px) activities, and with adiponectin levels. An in vitro culture of human adipocytes with 1 μM potassium iodide (KI, dose similar to the human breast milk iodine concentration) produced a significant decrease in adiponectin, GSH-Px, SOD1, and SOD2 mRNA expression. However, after 2 months of treatment with KI in the prospective study, a positive correlation was found between 24-h urinary iodine and serum adiponectin. Our observations lead to the hypothesis that iodine may be a factor directly involved in the regulation of oxidative stress and adiponectin levels in human breast milk. Antioxid. Redox Signal. 20, 847–853.
Introduction
An adequate concentration of iodine in breast milk is essential to provide for an optimal neonatal thyroid function and consequent normal neural development (8). The increasing awareness of the importance of an adequate iodine intake during pregnancy and lactation has led not just to the recommendation to consume iodized salt, but also to the prescription of iodide supplementation for pregnant and lactating women, especially in those areas where dietary iodine is even moderately deficient.
In addition to the synthesis of thyroid hormones, iodide has other roles. The role of iodine in mammary and other tissues has been shown to have an antioxidant function (3). Iodide was found to effectively scavenge reactive oxygen species (ROS) in human blood cells, decreasing damage by free oxygen radicals. In addition, iodide intake also seems to be related to the activity of the enzymes glutathione peroxidase (GSH-Px), superoxide dismutase (SOD), and catalase (3, 4). However, little information is available about the relation between the iodine status of lactating mothers and the antioxidant capacity of human breast milk.
Innovation.
Little is known about the association between iodine and human breast milk composition. In a study in lactating women, breast milk iodine was negatively associated with different oxidative stress markers and with adiponectin levels. An in vitro study with a potassium iodide dose similar to the concentration of human breast milk iodine produced a significant decrease in different oxidative stress markers and adiponectin. Iodine may be a factor directly involved in the regulation of oxidative stress and adiponectin levels in human breast milk.
Breast milk is composed of macro- and micronutrients specially suited to meet the needs of newborn infants. The recent identification in breast milk of obesity-related hormones, such as leptin, ghrelin, adiponectin, obestatin, and resistin, involved in the regulation of energy balance, suggests that breast milk could be a source of critical compounds in the metabolic development of the infant. No information exists on the possible association between iodine and these obesity-related hormones, and little is known about the association between breast milk iodine and oxidative stress.
We have previously shown the effect on the neuropsychological development of infants of a dietary supplementation with different doses of potassium iodide (KI) (8) and on the thyroid hormone, anti-inflammatory and antioxidant capacity of sera from adult subjects (4). However, as far as we are aware, no study has yet been undertaken to evaluate the effect of a KI supplement on the composition of human breast milk. Therefore, the aim of this study was to investigate the effect that a controlled increase of iodine in vivo and in vitro has on different markers of oxidative stress, lipid peroxidation, and obesity-related hormones in human breast milk.
Association Between Iodide and Adiponectin, SOD, GSH-Px, and Catalase
First, we analyzed the composition of human breast milk in a study undertaken in pregnant women. As Table 1 shows, iodine in breast milk was significantly lower in the control group (without iodine supplements during pregnancy) than in the 300 group (pregnant women with 300 μg of iodide [in the form of KI] per day from the first trimester of pregnancy and during lactation) (p=0.007). The iodine in breast milk correlated significantly with the urinary iodine in the mothers (r=0.261, p=0.025), but not with the urinary iodine in the newborns (r=0.165, p=0.119).
Table 1.
Variables of Mothers and Newborns in the Study of Human Breast Milk (Study A)
| Control group | 300 group | p-Value | |
|---|---|---|---|
| N | 67 | 21 | |
| Age of the mother (years) | 33.0±4.1 | 31.0±4.5 | 0.058 |
| Birth weight (kg) | 3.508±0.374 | 3.372±0.440 | 0.198 |
| Gestational age (weeks) | 40.1±1.0 | 39.2±1.3 | 0.002 |
| Urinary iodine in third trimester (μg/L) | 114.7±85.5 | 261.3±123.6 | 0.0001 |
| Urinary iodine in newborn (μg/L) | 112.3±57.5 | 215.2±163.0 | 0.010 |
| Breast milk iodine (μg/L) | 108.6±70.0 | 178.3±121.4 | 0.007 |
| Breast milk GSH-Px activity (nmol/min/ml) | 16.6±7.5 | 13.7±7.6 | 0.101 |
| Breast milk catalase activity (nmol/min/ml) | 24.8±14.4 | 26.6±29.0 | 0.200 |
| Breast milk SOD activity (U/ml) | 4.42±1.61 | 4.05±1.76 | 0.511 |
| Breast milk TBARs (μM) | 0.599±0.289 | 0.577±0.192 | 0.958 |
| Breast milk leptin (pg/ml) | 660.9±341.0 | 679.8±476.1 | 0.525 |
| Breast milk total ghrelin (pg/ml) | 174.7±53.5 | 216.3±108.0 | 0.130 |
| Breast milk adiponectin (ng/ml) | 47.4±38.2 | 32.6±36.9 | 0.060 |
| Breast milk obestatin (pg/ml) | 455.5±111.9 | 429.9±108.3 | 0.404 |
GSH-Px, glutathione peroxidase; SOD, superoxide dismutase; TBARS, thiobarbituric acid reactive substances.
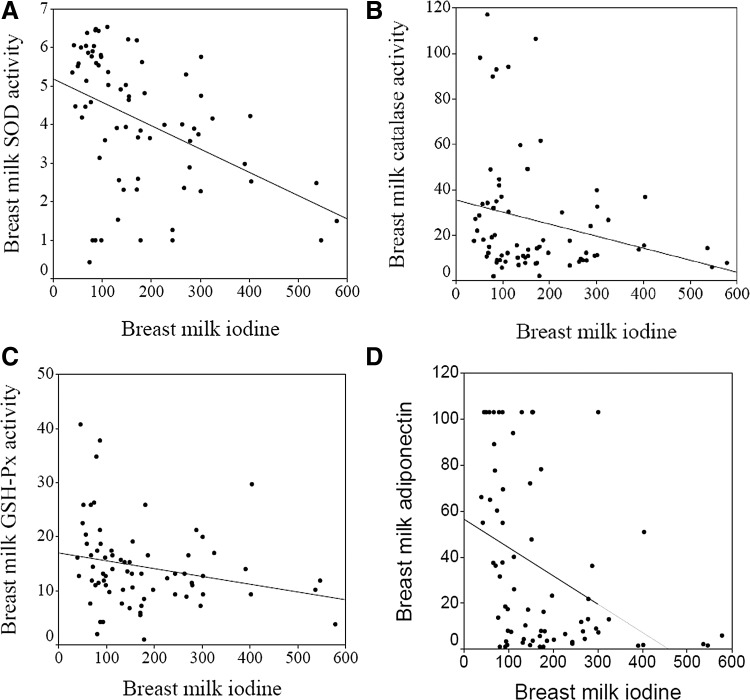
We have previously shown that ingestion of 100–300 μg iodide per day has a slight antioxidant effect in plasma (4). However, in other studies, the serum SOD activity was not affected by iodine supplementation, although the serum GSH-Px activity was significantly decreased. In our study, the oxidative stress variables and hormones analyzed were similar in the breast milk of the control group and the 300 group (Table 1). The iodine in breast milk correlated significantly with the activity of SOD (r=−0.459, p<0.0001) (Fig. 1A), catalase (r=−0.279, p=0.018) (Fig. 1B), and GSH-Px (r=−0.302, p=0.011) (Fig. 1C), and with adiponectin levels (r=−0.505, p<0.0001) (Fig. 1D). The iodine in breast milk did not correlate significantly with thiobarbituric acid reactive substances (TBARS), leptin, ghrelin, or obestatin levels (data not shown). The association between adiponectin and iodine remained significant (p=0.038) (R2=0.430) after adjusting for SOD, catalase, and GSH-Px activity and the body weight of the newborns. When we analyzed the control group and the 300 group separately, iodine in breast milk remained associated with adiponectin (control group: r=−0.584, p=0.014; 300 group: r=−0.411, p=0.002) and SOD activity (control group: r=−0.521, p=0.032; 300 group: r=−0.453, p=0.001). This negative association between iodine and the antioxidant activity in breast milk of different enzymes, together with studies showing that iodine can effectively scavenge ROS, suggests that iodine may also exert an antioxidant action in breast milk. Iodide is well suited as a versatile antioxidant.
FIG. 1.
Correlations between iodine and oxidative stress markers and adiponectin. The human breast milk iodine concentration (μg/L) correlated negatively with (A) SOD activity (U/ml) (r=−0.459, p<0.0001), (B) catalase activity (nmol/min/ml) (r=−0.279, p=0.018), (C) GSH-Px activity (nmol/min/ml) (r=−0.302, p=0.011), and (D) adiponectin levels (ng/ml) (r=−0.505, p<0.0001) in Study A. GSH-Px, glutathione peroxidase; SOD, superoxide dismutase.
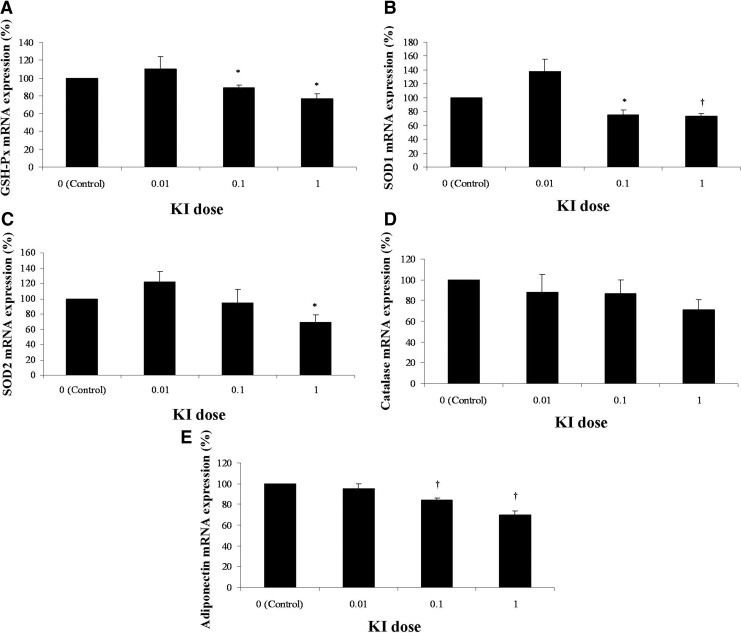
Given the associations between iodine and adiponectin and oxidative stress variables, we investigated whether iodide may have an effect on the catalase, GSH-Px, SOD, and adiponectin gene expression. Since adiponectin is produced mainly by adipocytes, we incubated human adipocytes obtained from preadipocytes of visceral adipose tissue (VAT) from lean subjects (n=5) with different doses of KI (0, 0.01, 0.1, and 1 μM) for 24 h. In the oxidative stress variables, 0.1 and 1 μM KI produced a significant decrease in GSH-Px (Fig. 2A) and SOD1 mRNA expression (Fig. 2B), while 0.01 μM KI had no significant effect. About 1 μM KI produced a significant decrease in SOD2 mRNA expression, while 0.01 and 0.1 μM KI had no significant effect (Fig. 2C). No significant effects were found with 0.01, 0.1, or 1 μM KI on catalase mRNA expression (Fig. 2D). This in vitro experiment, in which we showed that iodide levels similar to those found in breast milk (1 μM KI) can produce a decrease in the GSH-Px, SOD1, and SOD2 expression in human mature adipocytes, corroborates the association found in breast milk. The high levels of iodine in breast milk can produce a decrease in the ROS and H2O2 levels. Because these products represent the specific substrate for SOD, catalase, and GHS-Px, a decrease of these substrates may result in a lower level of these antioxidant enzymes. However, a 0.01 μM dose, similar to the normal serum iodide levels (2), did not produce a significant change in the expression of these antioxidant enzymes.
FIG. 2.
Effect of iodide on oxidative stress markers and on adiponectin expression levels (Study B). Adipocytes obtained from preadipocytes isolated from human visceral adipose tissue (n=5) were incubated in the presence of 0 (control dose), 0.01, 0.1, and 1 μM of iodide in the form of KI for 24 h. Following these treatments, adipocytes were collected and mRNA extracted. (A) GSH-Px mRNA expression levels. (B) SOD1 mRNA expression levels. (C) SOD2 mRNA expression levels. (D) Catalase mRNA expression levels. (E) Adiponectin mRNA expression levels. The results are expressed as a percentage of control levels (0 dose) (100%). Data are the mean±standard error of five separate experiments. Three replicate wells were evaluated per treatment in each experiment. *p<0.05, †p<0.01: significant differences from the corresponding control group without KI treatment. KI, potassium iodide.
Given the association between iodine and adiponectin in human breast milk, we also investigated whether iodide may have an effect on the adiponectin expression. About 0.1 and 1 μM KI produced a significant decrease in adiponectin mRNA expression, while 0.01 μM KI had no significant effect (Fig. 2E). Previous in vitro studies suggest that antioxidants can regulate adiponectin expression through a reduction in oxidative stress. In our case, we found a strong and negative relation between iodine and adiponectin levels in breast milk. This negative association was confirmed by the in vitro experiment. This association is unexpected. Doses similar to the iodide concentration in human breast milk, but 10 and 100 times higher than the normal iodide plasma levels, produced a significant decrease in the adiponectin expression levels. At doses similar to the normal serum iodide levels (2), iodide did not produce any effect on the adiponectin levels.
As the association between iodine and adiponectin levels in breast milk was unexpected, we wished to determine whether this relationship also existed between urinary iodine levels and serum adiponectin. We have analyzed this association in two previous studies. In the first study (Pizarra Study) (5), we selected the subjects with normal glucose metabolism to avoid interference by a carbohydrate metabolism disorder, which may alter the adiponectin levels. In these subjects, the mean serum adiponectin concentration was 11.5±6.1 ng/ml, and the mean urinary iodine concentration was 129.9±98.4 μg/L. We found no significant correlation between urinary iodine and serum adiponectin (r=−0.001, p=0.997). No significant correlations were found after adjusting for glucose levels (r=0.009, p=0.857).
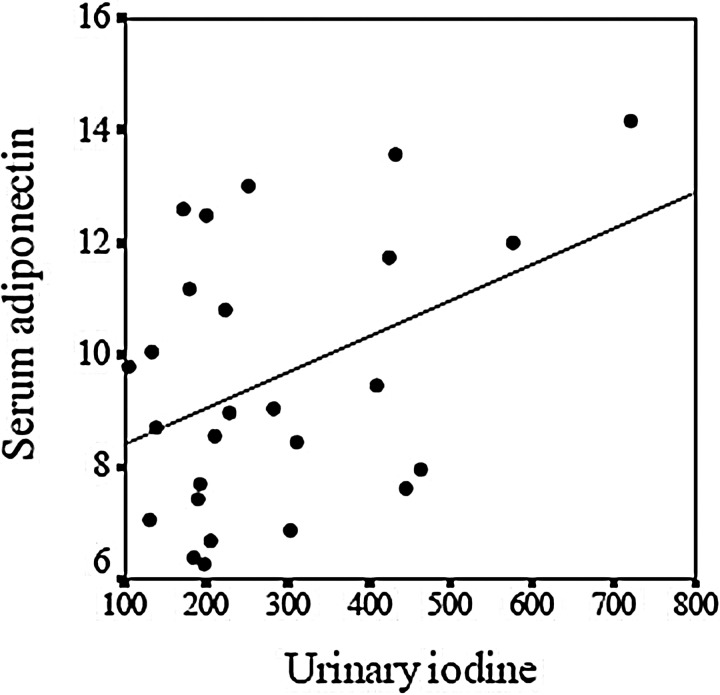
The second study was carried out in thirty healthy volunteers, with a normal thyroid function, supplemented with a daily dose of 100, 200, or 300 μg KI for 2 months, doses similar to those usually recommended in clinical practice for the prevention of iodine deficiency in pregnant women (4). In these subjects, the mean baseline serum adiponectin concentration was 10.7±4.1 ng/ml, and the mean baseline urinary iodine concentration was 173.8±73.8 μg/L in the casual samples and 180.1±76.8 μg/day in the 24-h urinary samples, with no significant differences between the three groups of subjects (100, 200, and 300 groups). No significant correlations were found between the baseline serum adiponectin and urinary iodine concentrations, either in the casual (r=−0.095, p=0.650) or the 24-h urinary samples (r=−0.062, p=0.763). Neither were significant correlations found after adjusting for glucose levels (r=−0.065, p=0.750 and r=−0.064, p=0.589, respectively). After 2 months of treatment, the urinary iodine concentration increased significantly in the casual samples (4) and in the 24-h urinary samples (100 group: 191.6±90.3 vs. 223.3±91.7 μg/day, p=0.063; 200 group: 140.3±74.6 vs. 230.4±130.9 μg/day, p=0.032, and 300 group: 200.6±56.4 vs. 377.0±179.4 μg/day, p=0.011). Serum adiponectin did not change significantly in the three groups, either separately or in all the subjects together (data not shown). After these 2 months of treatment, significant correlations were found between serum adiponectin and urinary iodine in 24-h urinary samples after adjusting for glucose levels (r=0.404, p=0.048) (Fig. 3), but not between serum adiponectin and urinary iodine in casual samples (r=−0.046, p=0.823). This different behavior of iodine in serum and in breast milk may be due to various causes. The breast concentrates iodine in higher levels than serum and this could have different effects; the in vitro inhibition of adiponectin expression by iodide is observed with doses similar to breast milk iodine levels, but not with iodide doses similar to serum levels. On the other hand, low iodine doses can have a slight and chronic antioxidant effect, as we have demonstrated in a previous study (4). However, the relation between iodine and adipokines is not well known.
FIG. 3.
Correlation between serum adiponectin (ng/ml) and urinary iodine in 24-h urinary samples (μg/day) after 2 months of treatment with KI in the prospective study (Study D), after adjusting for glucose levels (r=0.404, p=0.048).
These findings may have different implications. On the one hand, several arguments suggest that the adiponectin in human milk could be biologically relevant, although this remains under question. Higher adiponectin concentrations in human milk appear to be associated with lower infant weight in the first 6 months of life (1). However, another study showed that high levels of adiponectin in maternal milk may be a risk factor for childhood overweight at the age of 2 years (9). It is possible that the effects observed to be associated with milk adiponectin could be caused by other related factors, such as other milk proteins whose synthesis is linked with that of adiponectin, or adiponectin in combination with other factors (1). In addition, the presence of adiponectin receptor 1 in fetal small intestine suggests that a direct role of oral adiponectin in the neonate is possible. In this context, the iodine level may have importance and further research is needed. However, in spite of the negative correlation with adiponectin, breast milk iodine is the main source of iodine intake by the infant. An adequate iodine intake is necessary because iodine will be mainly used for the synthesis of thyroid hormones in the infant.
This study has certain strengths. It comprised cross-sectional, prospective and in vitro studies. Data obtained from the in vitro study corroborate the observational findings in human breast milk. Additionally, dietary iodine supplements at the doses used here not only have any harmful effects on serum adiponectin levels, they may even be beneficial. However, our results and their interpretation are limited because we did not measure other antioxidants or pro-oxidants or maternal factors, including nutritional status and smoking, which may affect the antioxidant status.
Concluding Remarks and Future Directions
To the best of our knowledge, our data are the first to show that iodine is associated significantly with oxidative stress markers and adiponectin in human breast milk. The decrease in the oxidative stress variables in human breast milk can reflect an increase in their antioxidant capacity due to an increase in the iodine content. More experiments are needed to investigate the role of iodine in the antioxidant status. In addition, iodine can thus be suggested as a factor directly involved in the regulation of adiponectin levels, at least in human breast milk. However, the mechanisms of adiponectin modulation by iodide are unclear, and more studies are needed.
Notes
Study subjects
This work includes different studies. These studies were conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures were approved by the Ethics and Research Committees of Carlos Haya University Hospital. Written informed consent was obtained from all subjects.
Study A: study of human breast milk
This study was undertaken in 88 pregnant women from the Hospital de la Merced (Osuna, Andalusia, and southern Spain) (8). A total of 67 consecutive women were given 300 μg of iodide (in the form of KI) from the first trimester of pregnancy (300 group). The women continued taking the same dose during lactation. The control group (n=21) was selected from among those women who attended the Osuna Hospital during the last month of pregnancy and who had not received iodine supplements (iodized salt, KI, or any other iodine-enriched supplement).
Study B: adipocyte culture
As some hormones found in human breast milk are adipocytokines, we performed an in vitro experiment to determine the effect of different doses of KI on the adiponectin, GSH-Px, SOD, and catalase expression in adipocytes. VAT was obtained during intra-abdominal surgery (cholecystectomy) from five nonobese subjects with normal glucose levels (two male, three female; age 38.1±5.2 years, body mass index [BMI] 25.0±3.3 kg/m2, fasting glucose 85.5±9.9 mg/dl).
Studies C and D: studies of urinary iodine and serum adiponectin
This report combines two studies previously undertaken by the authors in southern Spain. The first (Study C), the Pizarra Study, is a population-based prospective study undertaken in a population from Andalusia, southern Spain. The study population and the design of this survey have been described previously (5). From the original cohort (1996–1998), 784 (83.6%) individuals were reassessed in 2002–2004. Of this study, we selected the subjects reassessed in 2002–2004 who had normal glucose levels, both at baseline and after an oral glucose tolerance test, and in whom adiponectin levels were analyzed (n=508, 64.8%) (BMI: 27.2±4.7 kg/m2).
The second study (Study D) was undertaken in 30 healthy volunteers (10 men and 20 women) (BMI: 24.7±3.1 kg/m2) with normal glucose levels, both at baseline and after an oral glucose tolerance test. The study population and the design have been described previously (4). The subjects, who commonly consumed iodized salt as part of their usual daily diet, were assigned to one of the three groups (n=10). Each group received a daily dose of 100, 200, or 300 μg of KI for 2 months.
Procedures (Studies A, C, and D)
In the study undertaken in pregnant women (Study A), measurements were made of urinary iodine concentrations from a casual sample of urine during the third trimester of pregnancy and in the newborns. Breast milk samples were collected between 0 and 1 day after delivery. Breast milk was collected in the morning at fasting status in sterile tubes using a manual breast pump. An aliquot was frozen immediately after collection and stored at −20°C until iodine analysis. Within 2 h of collection, a breast milk aliquot was centrifuged for 20 min, 2500 g at 4°C. The fat layer and cellular compartments were removed and the aqueous phase was collected and stored at −80°C until analysis for oxidative stress markers and hormones. Leptin, ghrelin, adiponectin, obestatin, GSH-Px activity, SOD activity, catalase activity, and TBARS (an indicator of lipid peroxidation) were measured in the aqueous phase of breast milk.
In the Pizarra Study (Study C), measurements were made of urinary iodine concentrations in a casual sample of urine and of serum adiponectin in samples previously aliquoted and frozen at −80°C.
In Study D, measurements were made of urinary iodine concentrations in casual and in 24-h urinary samples, and of serum adiponectin in samples previously aliquoted and frozen at −80°C, both at baseline and after 2 months of treatment.
The iodine concentration in urine and human milk samples was measured by the modified Benotti and Benotti technique (4, 8). The intra- and interassay coefficients of variation of the urinary iodine assay were 2.01% and 4.53%, respectively. In addition, the urinary iodine assay is subjected to a program of external quality assessment for the determination of iodine in urine of the Spanish Association of Neonatal Screening (AECNE). The reference material was Seronorm™ Trace Elements Urine, with a mean z-score of 0.3. GSH-Px, SOD, and catalase activity were measured in human milk using commercial kits (Cayman Chemical Company). TBARS were determined by spectrophotometry as previously described (7). Ghrelin (total ghrelin) was assayed by a sandwich enzyme-linked immunosorbent assay (ELISA) using a commercial kit (Millipore Corporation). Leptin levels were assayed by a commercial ELISA kit (R&D Systems Europe Ltd.). Adiponectin levels were assayed by a commercial ELISA kit (Mediagnost). Obestatin levels were assayed by a commercial radioimmunoassay kit (Phoenix Europe GmbH).
Adipocyte culture (Study B)
Preadipocyte culture and differentiation
All reagents were from Sigma-Aldrich (Sigma-Aldrich) unless otherwise specified. Fat tissue was digested in the Dulbecco's modified Eagle's medium (DMEM) with 1 mg/ml of collagenase buffer (Worthington Biochemical Corporation) and 10% fetal bovine serum (FBS) in a 37°C shaking water bath. Digests were filtered and centrifuged at 300 g for 10 min. Digests were treated with an erythrocyte lysis buffer. Cells were plated in the DMEM-Ham's F-12 supplemented with 10% FBS, 1% l-glutamine, 1% penicillin, and streptomycin. The plating medium was changed every 2 days until confluence. Newly confluent cultures were subcultured in 24-well plates, and grown to confluence in the DMEM-Ham's F-12 supplemented with 10% FBS, 1% l-glutamine, 1% penicillin, and streptomycin. At this stage, designated as day 0, differentiation was induced by treatment with StemXVivo™ Osteogenic/Adipogenic Base Media with StemXVivo Adipogenic Supplement (R&D Systems, Inc.) for the differentiation of human mesenchymal stem cells into adipocytes. The medium was changed every 2 days until day 15. On this day, the medium was replaced with a fresh medium supplemented with 0, 0.01 [dose similar to that found in human serum (2)], 0.1, and 1 μM [dose similar to that found in human breast milk (8)] of iodide in the form of KI, for 24 h. Following these treatments, adipocytes were collected and mRNA extracted. Each treatment was performed in triplicate.
RNA extraction and real-time quantitative polymerase chain reaction
Total RNA was extracted by RNeasy lipid tissue midi kit (QIAGEN Science) as previously described (6). Total RNA was reverse transcribed to cDNA by using a high-capacity cDNA reverse transcription kit with RNase inhibitor (Applied Biosystems). The cDNA was used for quantitative real-time polymerase chain reaction (PCR) with duplicates. We analyzed the mRNA expression levels of human adiponectin, catalase, GSH-Px, SOD1, and SOD2. The primers used were designed based on NCBI database sequences and obtained from Proligo. The primers used were adiponectin (Fwd: 5′-CCTAAGGGAGACATCGGTGA-3′; Rev: 5′-GTAAAGCGAATGGGCATGTT-3′) (NC_000003.11), catalase (Fwd: 5′-GCCTGGGACCCAATTATCTT-3′; Rev: 5′-GAATCTCCGCACTTCTCCAG-3′) (NM_001752.3), GSH-Px (Fwd: 5′-CCAGTCGGTGTATGCCTTCT-3′; Rev: 5′-CTGCAGCTCGTTCATCTGG-3′) (NM_000581.2), SOD1 (Fwd: 5′-AGGGCATCATCAATTTCGAG-3′; Rev: 5′-ACATTGCCCAAGTCTCCAAC-3′) (NM_000454.4), SOD2 (Fwd: 5′-GGAAGCCATCAAACGTGACT-3′; Rev: 5′-CCTTGCAGTGGATCCTGATT-3′) (NM_001024465.1, NM_001024466.1, and β-actin (internal control) (Fwd: 5′-TACAGCTTCACCACCACGGC-3′; Rev: 5′-AAGGAAGGCTGGAAGAGTGC-3′) (NM_001101). The amplifications were performed on an ABI 7500 Fast Real-Time PCR System (Applied Biosystems). Real-time quantitative PCR reactions were carried out in a 20-μl reaction mixture containing the cDNA along with primers for each transcript and KAPA SYBR® FAST Universal 2×qPCR Master Mix (Kapa Biosystems, Inc.). Melting curve analyses were performed to ensure that only a single product was amplified. There is no variation in the expression of this housekeeping gene (β-actin) in the condition tested. The cycle threshold value for each sample was normalized with the expression of β-actin.
Statistical study
The data are presented as the mean±standard deviation. Comparisons of two samples were made with the Mann–Whitney test. Comparisons of more than two samples were done with the Kruskal–Wallis test. The correlation between variables was determined using the Spearman test, and by multiple linear regression models. In all cases, the level of rejection of a null hypothesis was an α ≤0.05 for two tails.
Abbreviations Used
- BMI
body mass index
- DMEM
Dulbecco's modified Eagle's medium
- ELISA
enzyme-linked immunosorbent assay
- FBS
fetal bovine serum
- GSH-Px
glutathione peroxidase
- KI
potassium iodide
- PCR
polymerase chain reaction
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- TBARS
thiobarbituric acid reactive substances
- VAT
visceral adipose tissue
Acknowledgments
This work was supported, in part, by a grant from the Instituto de Salud Carlos III (PS09/01060). CIBER de Diabetes y Enfermedades Metabólicas Asociadas and CIBER de Fisiopatología de la Obesidad y Nutrición are ISCIII initiatives. We also thank Ian Johnstone for help with the English language version of the text.
References
- 1.Newburg DS, Woo JG, and Morrow AL. Characteristics and potential functions of human milk adiponectin. J Pediatr 156: S41–S46, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Panneels V, Juvenal G, Boeynaems JM, Dumont JE, and Van Sande J. Iodide effects on the thyroid: biochemical, physiological, pharmacological, and clinical effects of iodide in the thyroid. In: Comprehensive Handbook of Iodine, Nutritional, Biochemical, Pathological and Therapeutic Aspects, edited by Preedy VR, Burrow GN, and Watson RR. San Diego, CA: Academic Press, 2009, pp. 303–314 [Google Scholar]
- 3.Smyth PP. Role of iodine in antioxidant defence in thyroid and breast disease. Biofactors 19: 121–130, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Soriguer F, Gutiérrez-Repiso C, Rubio-Martin E, Linares F, Cardona I, López-Ojeda J, Pacheco M, González-Romero S, Garriga MJ, Velasco I, Santiago P, and García-Fuentes E. Iodine intakes of 100–300 μg/d do not modify thyroid function and have modest anti-inflammatory effects. Br J Nutr 25: 1–8, 2011 [DOI] [PubMed] [Google Scholar]
- 5.Soriguer F, Valdes S, Morcillo S, Esteva I, Almaraz MC, de Adana MS, Tapia MJ, Dominguez M, Gutierrez-Repiso C, Rubio-Martin E, Garrido-Sanchez L, Perez V, Garriga MJ, Rojo-Martinez G, and Garcia-Fuentes E. Thyroid hormone levels predict the change in body weight: a prospective study. Eur J Clin Invest 41: 1202–1209, 2011 [DOI] [PubMed] [Google Scholar]
- 6.Tinahones FJ, Garrido-Sanchez L, Miranda M, García-Almeida JM, Macias-Gonzalez M, Ceperuelo V, Gluckmann E, Rivas-Marin J, Vendrell J, and García-Fuentes E. Obesity and insulin resistance-related changes in the expression of lipogenic and lipolytic genes in morbidly obese subjects. Obes Surg 20: 1559–1567, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Tinahones FJ, Murri-Pierri M, Garrido-Sánchez L, García-Almeida JM, García-Serrano S, García-Arnés J, and García-Fuentes E. Oxidative stress in severely obese persons is greater in those with insulin resistance. Obesity (Silver Spring) 17: 240–246, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Velasco I, Carreira M, Santiago P, Muela JA, García-Fuentes E, Sánchez-Muñoz B, Garriga MJ, González-Fernández MC, Rodríguez A, Caballero FF, Machado A, González-Romero S, Anarte MT, and Soriguer F. Effect of iodine prophylaxis during pregnancy on neurocognitive development of children during the first two years of life. J Clin Endocrinol Metab 94: 3234–3241, 2009 [DOI] [PubMed] [Google Scholar]
- 9.Weyermann M, Brenner H, and Rothenbacher D. Adipokines in human milk and risk of overweight in early childhood: a prospective cohort study. Epidemiology 18: 722–729, 2007 [DOI] [PubMed] [Google Scholar]