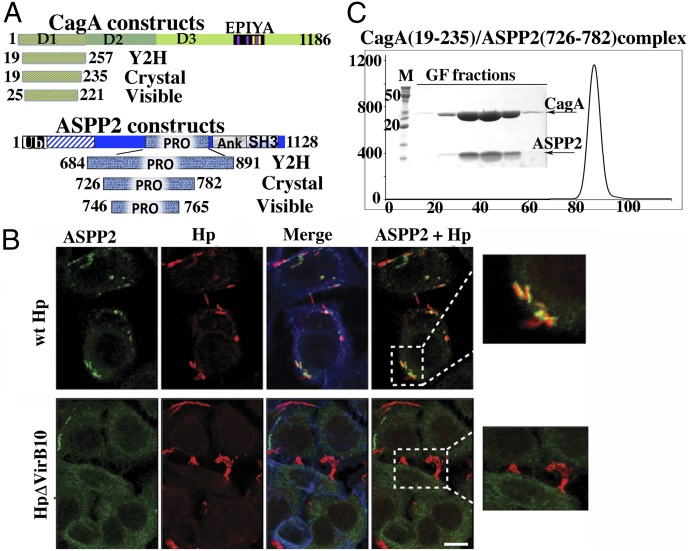
Fig. 1.
ASPP2 binding and recruitment by CagA. (A) Domain delineation of a CagA–ASPP2 minimal complex. The schematic diagram of constructs of CagA and ASPP2 used in this study are as follows: Crystal, constructs that were coexpressed in E. coli, copurified, and used in crystallization trials; Visible, domains that are visible in crystal structure; Y2H, constructs used in Y2H screen. (B) Relocalization of endogenous ASPP2 during the course of Hp infection is CagA dependent. AGS cells were infected with the indicated Hp strains (moi 1:50) or left uninfected. After 7 h, cells were fixed and stained with anti-ASPP2 (green) and anti-CagA (red) antibodies and phalloidin for F-actin (blue). Insets show that only wild-type Hp (wt Hp, Upper Inset) strongly associates with ASPP2, whereas there is no association with Hp ΔVirb10 mutant (Lower Inset). (Scale bar: 10 μm.) (C) Gel filtration profile of CagA (19–235) and ASPP2 (726–782) complex. The final step of purification of CagA (19–235) and ASPP2 (726–782) complex used in crystallization experiments is shown. The elution profile from gel filtration column (Superdex 200; GE Health) and SDS/PAGE of peak fractions are presented.