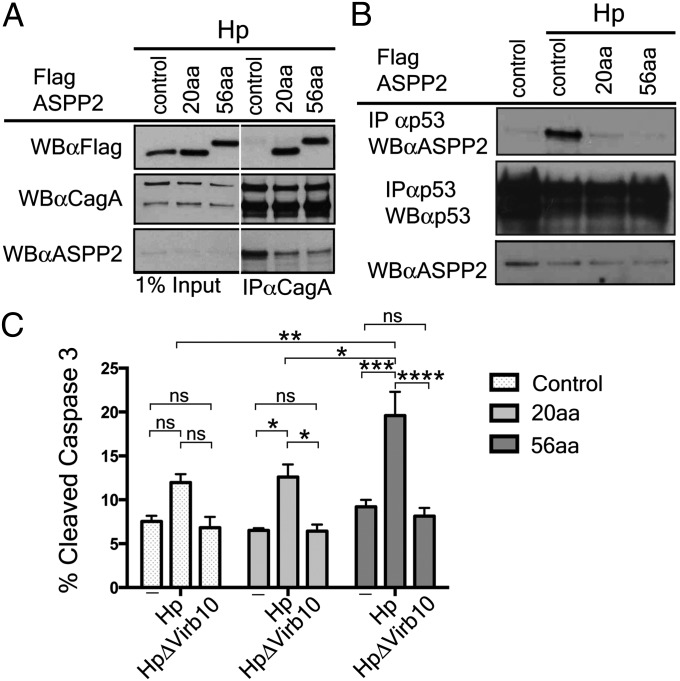
Fig. 4.
Expression of the CagA-binding domain of ASPP2 blocks the downstream pathway activated by delivery of CagA. (A) The CagA-binding domain of ASPP2 is sufficient to bind to CagA during Hp infection and inhibit interaction with endogenous ASPP2. AGS cells stably expressing FLAG-mCherry, FLAG-mCherry ASPP2 56aa, or FLAG-mCherry-ASPP2 20aa were infected with wild-type Hp (moi 1:50). Seven hours after infection, cells were lysed, and lysates were immunoprecipitated or directly immunoblotted with the indicated antibodies. CagA was retrieved with an anti-CagA antibody. Immunoprecipitates were separated by SDS/PAGE and detected by immunoblotting using the indicated antibodies. (B) Dominant negative effect of the CagA-binding domain of ASPP2 on the ASPP2–p53 interaction following Hp infection. AGS cells stably transfected with the indicated constructs were infected with wt Hp or left uninfected. Seven hours after infection, cells were harvested and processed as indicated in A. Uninfected cells were also used as a control. (C) Increased apoptosis of Hp-infected cells upon disruption of CagA–ASPP2 binding. Levels of cleaved Caspase-3 were assayed as a measure of apoptotic response by flow cytometry. AGS cells stably transfected with the indicated constructs (control, 20aa, and 56aa), were infected for 24 h with the indicated Hp strains (moi 1:50) or left uninfected. Error bars ± SEM (n = 4); ns, not significant; ****P < 0.0001; ***P < 0.001; **P < 0.01;*P < 0.05. Significance was tested by using two-way ANOVA Tukey multiple-comparison test.