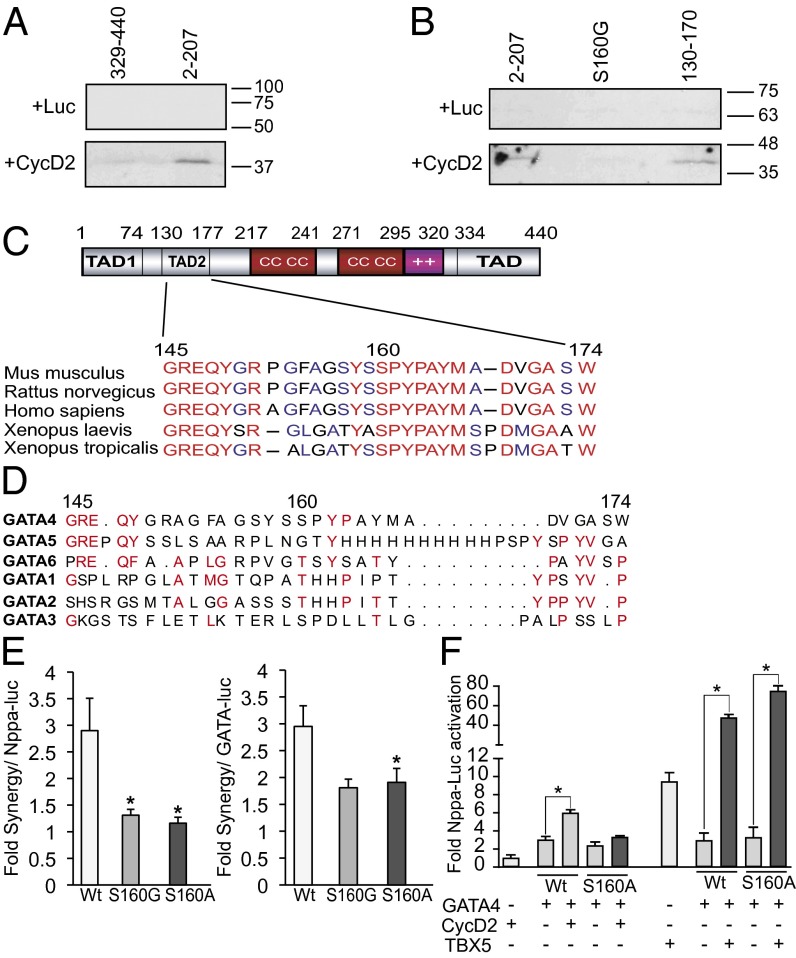
Fig. 3.
CycD2 physically interacts with N-terminal GATA4 in vitro. (A) In vitro-translated radiolabeled CycD2 protein (or luciferase protein as a negative control) was incubated with glutathione Sepharose beads containing GST–N-terminal GATA4 or GST–C-terminal GATA4 fusion proteins. The bound proteins were then resolved by SDS/PAGE and revealed by autoradiography. Note that CycD2 binds to N-terminal GATA4 (second lane). The experiment is one representative of two. (B) Amino acids 130–170 are sufficient to interact with CycD2, and S160 is required for this interaction. In vitro-translated radiolabeled CycD2 protein were incubated with glutathione Sepharose beads containing GST alone, GST–N-terminal region 2–207 of GATA4, the same GST–N-terminal part of GATA4 harboring the S160G mutation or GST–130–170 GATA4 fusion proteins. The bound proteins were then resolved by SDS/PAGE and revealed by autoradiography. In vitro-translated luciferase protein was used as a negative control. Note that CycD2 binds to amino acids 2–207 of GATA4 and 130–170 but not S160G mutant. The experiment is one representative of two. (C) Schematic representation of GATA4 protein. Note that the 145- to 174-aa region, and particularly amino acid S160, is highly conserved among the mouse, rat, human, and Xenopus. (D) Alignment of the CycD2-interacting domain (145–174) of human GATA4 with the other GATA members as indicated. Note that this region is highly divergent among the different GATA members. (E) Fold synergy of CycD2 with WT (Wt) GATA4 or the indicated S160 GATA4 mutants on Nppa promoter (Left) or GATA-dependent promoter (Right). Note the reduced fold of synergy with both S160G and S160A. *P < 0.05 vs. Wt. (F) Transient transactivation of Nppa promoter by WT or S160A GATA4 with or without either CycD2 or TBX5; 250 ng of GATA4 was used with 4 μg of CycD2; 5 ng of GATA4 was used with 50 ng of TBX5. Note the S160 mutation attenuates synergy with CycD2 but not TBX5. *P < 0.05 vs. GATA4.