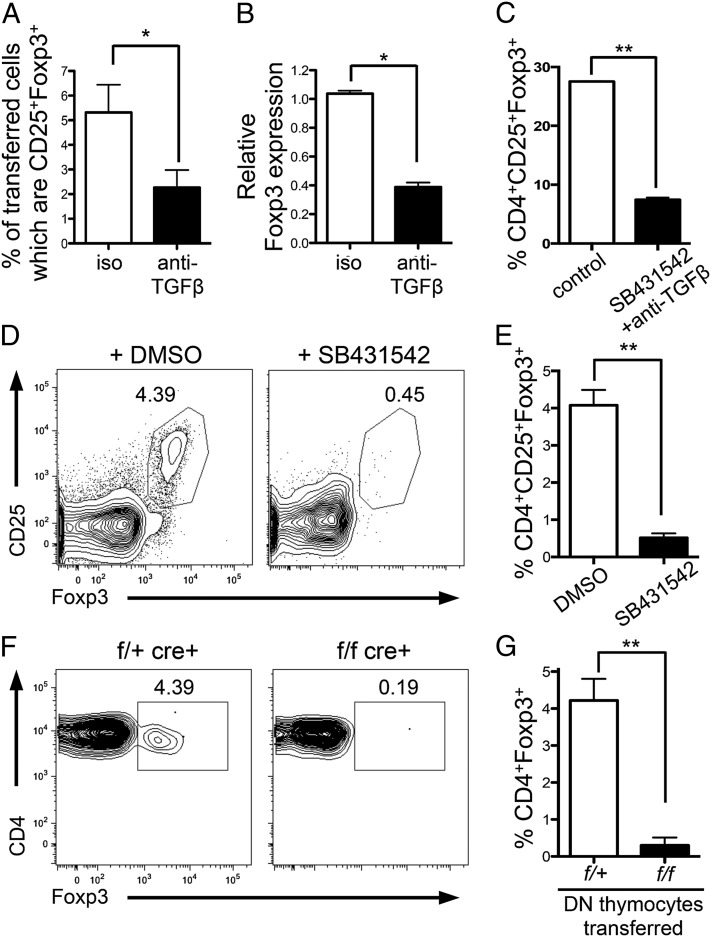
Fig. 1.
TGF-β drives tTreg generation. (A and B) CD4+CD25− DO11.10xRag−/− thymocytes were i.t.-transferred to BALB/c mice with 1 μg pOVA plus 150 μg anti-TGFβ or isotype control. (A) Thymi were harvested after 5 d, and Foxp3 expression in the transferred population was examined by FACS or (B) after 18 h when transferred cells were FACS-sorted and foxp3 expression examined by quantitative PCR (qPCR). Data represent three to five experiments. (C) CD4+CD25−GFP− SP thymocytes were FACS-sorted and cultured with anti-CD3+anti-CD28 for 18 h followed by 36 h rest in the presence or absence of anti-TGFβ and TGFβ inhibitor (SB431542) or isotype control and DMSO. Bar graph shows frequency of CD25+Foxp3+ cells in cultures. Data represent two experiments. (D and E) FTOC cultures were established with E16.5 fetal lobes and cultured for 1 wk with TGFβ inhibitor (SB431542) or DMSO control. (D) Representative FACS plots gated on CD4+SP thymocytes. (E) Bar graph showing frequency of CD25+Foxp3+ cells. Data represent three experiments. (F and G) FACS-sorted DN thymocytes from Tgfbr1+/fCD4-cre+ (control; f/+) or Tgfbr1f/fCD4-cre+ (KO; f/f) mice were transferred i.t. into congenic C57BL/6 mice. Thymi were harvested after 12 d. (F) Representative FACS plots gated on transferred CD4+SP thymocytes. (G) Bar graph showing frequency of Foxp3+ cells in transferred CD4+SP thymocytes. Data represent five experiments. Error bars represent mean ± SEM. *P < 0.05, **P < 0.005 (unpaired two-tailed Student t test).