Abstract
Solithromycin (CEM-101) is a new antibiotic that is highly potent against Ureaplasma and Mycoplasma spp. and active against many other antibiotic-resistant organisms. We have explored the maternal-amniotic-fetal pharmacokinetics of CEM-101 in a pregnant sheep model to assess its potential for treating intrauterine and antenatal infection. Chronically catheterized pregnant ewes (n = 6 or 7) received either a single maternal intravenous (i.v.) infusion of CEM-101 (10 mg/kg of body weight), a single intra-amniotic (i.a.) injection (1.4 mg/kg of estimated fetal weight), or a combined i.v. and i.a. dose. Maternal plasma (MP), fetal plasma (FP), and amniotic fluid (AF) samples were taken via catheter at intervals of 0 to 72 h postadministration, and concentrations of solithromycin and its bioactive polar metabolites (N-acetyl [NAc]–CEM-101 and CEM-214) were determined. Following maternal i.v. infusion, peak CEM-101 concentrations in MP, FP, and AF were 1,073, 353, and 214 ng/ml, respectively, representing a maternal-to-fetal plasma transfer efficiency of 34%. A single maternal dose resulted in effective concentrations (>30 ng/ml) in MP, FP, and AF sustained for >12 h. NAc–CEM-101 and CEM-214 exhibited delayed accumulation and clearance in FP and AF, resulting in an additive antimicrobial effect (>48 h). Intra-amniotic solithromycin injection resulted in elevated (∼50 μg/ml) and sustained CEM-101 concentrations in AF and significant levels in FP, although the efficiency of amniotic-to-fetal transfer was low (∼1.5%). Combined i.v. and i.a. administration resulted in primarily additive concentrations of CEM-101 in all three compartments. Our findings suggest that CEM-101 may provide, for the first time, an effective antimicrobial approach for the prevention and treatment of intrauterine infection and early prevention of preterm birth.
INTRODUCTION
Intrauterine infection and inflammation play a well-recognized role in the etiology of spontaneous preterm labor and birth, particularly in deliveries less than 32 weeks of gestation or those complicated by preterm prelabor rupture of membranes (PPROM) (1, 2). The origin of the infection is typically the vaginal flora: microorganisms are hypothesized to breach the cervical barrier, infect the fetal membranes, and eventually colonize the amniotic cavity (2–4). The vigorous inflammatory response that ensues is responsible for activation of myometrial contractions, membrane degradation, and rupture and cervical ripening, leading to labor and delivery (2, 5, 6). Intracellular organisms of the Mollicutes class, namely, Ureaplasma and Mycoplasma species, are the microorganisms most commonly isolated from the amniotic fluid (AF) of preterm deliveries (7, 8) and have been shown to be capable of eliciting preterm labor via a robust intrauterine inflammatory response in a dose-dependent fashion (9–11). Numerous other bacterial classes have also been identified in infected amniotic fluid samples, including streptococci, staphylococci, enterococci, Fusobacterium spp., Bacteroides spp., and Haemophilus spp. (8, 12).
A variety of clinical trials of maternal antibiotic administration have been performed to attempt to prevent or treat intrauterine infection with the aim of reducing the rates of preterm birth and associated neonatal morbidities; however, the benefits of these interventions have been unconvincing (13). The conclusions reached by the authors of several recent meta-analyses are that there is no evidence that treatment of women at risk of infection-driven preterm birth with antibiotics prophylactically or upon presentation with preterm labor reduces the rates of preterm delivery or improves neonatal outcomes (14–16). Not all researchers are in agreement, however. In some studies in which antibiotics (primarily clindamycin) have been administered to high-risk women prior to 22 weeks of gestation, significant reductions in rates of delivery before 37 and 33 weeks and in late miscarriage have been documented (17).
The reasons for the generally disappointing results of antibiotic interventions are likely severalfold, but the choice of antibiotic is a key factor. Macrolide antibiotics such as erythromycin and azithromycin are widely prescribed during pregnancy for the treatment of a variety of microbial infections, as they are perceived to be well tolerated, effective in treating important microorganisms such as Ureaplasma and Mycoplasma spp., and free of serious maternal and fetal side effects (18–21). Erythromycin is the most frequently administered antibiotic for treatment of PPROM based on the findings of the ORACLE I trial (22). However, there is strong evidence that systemic maternal erythromycin administration is largely ineffective in eradicating intrauterine Ureaplasma species infection (23). In an experimental sheep model of intrauterine Ureaplasma species colonization during pregnancy, we have shown that maternal intramuscular (i.m.) erythromycin administration does not eradicate Ureaplasma species infection from the amniotic fluid, chorioamnion, umbilical cord, or fetal lung (24). This is likely attributable to poor transplacental/transamniotic passage of macrolides. In the ex vivo perfused human placenta model, the transfer rate of macrolides is only 2 to 4% (25).
We recently showed that in sheep, maternal macrolide administration fails to deliver effective chemotherapeutic levels to either the fetal circulation or the amniotic cavity (26). In vivo studies confirm that the degree of erythromycin passage to the human fetus is low and variable (27, 28), while the extent of maternal-to-amniotic fluid transfer is more uncertain. A recent study in pregnant women at term reported that the transfer of azithromycin from maternal circulation to AF was more efficient than the maternal-to-fetal plasma transfer, although resulting AF concentrations (∼150 ng/ml) still failed to reach the MIC90 for Ureaplasma spp. (∼500 ng/ml) (29). Interestingly, a recent study in pregnant primates with intra-amniotic (i.a.) Ureaplasma infection showed that a sustained 10-day maternal course of azithromycin (12.5 mg/kg of body weight) achieved effective antimicrobial levels in the amniotic fluid and low levels in the fetal circulation (30); however, although the infection was cleared in 90% of animals, intra-amniotic and fetal inflammation remained evident following azithromycin treatment, and pregnancy length was extended by only 7 to 10 days, with all animals delivering preterm (30). From these studies, it is clear that intrauterine infections are difficult to eradicate and a more potent antibiotic with better maternal-amniotic-fetal transfer properties is required to eliminate both fetal and amniotic infection, in combination with an effective anti-inflammatory therapeutic to prevent the adverse consequences of inflammation within the amniotic cavity.
Solithromycin (CEM-101) is a novel macrolide/fluoroketolide antibiotic which exhibits broad-spectrum activity against Gram-positive and some Gram-negative organisms, including Neisseria gonorrhoeae, Chlamydia trachomatis, Haemophilus spp., streptococci, and enterococci (31–37). It is exceptionally potent against Ureaplasma parvum, Ureaplasma urealyticum, and Mycoplasma hominis (125 to 250 times more potent than azithromycin) (31). The fluoro group incorporated into its structure imparts activity against even highly resistant strains of multiple classes of microorganisms (38). It is acid stable with excellent oral bioavailability and demonstrates excellent tissue uptake and accumulation (39); it is also bactericidal at concentrations 2 to 8 times its MIC90 for some pathogens (40). Solithromycin has a plasma half-life (t1/2) in humans of approximately 7 h and has two polar phase I metabolites, both of which are bioactive, although there are significant species differences in the extent of metabolism (41, 42). Currently in clinical trials (43), solithromycin appears to be well tolerated and free of the adverse effects associated with its ketolide predecessor telithromycin (43, 44).
Due to its excellent spectrum of activity against a wide range of susceptible and resistant microorganisms, we believe solithromycin may represent a significant therapeutic advance for the treatment and prevention of intrauterine infections, depending on its ability to cross the placenta and fetal membranes and reach the fetus and amniotic cavity. The aim of the present study, therefore, was to determine the pharmacokinetics and maternal-to-fetal plasma transfer of solithromycin in a pregnant ovine model to assess its potential for treating intrauterine and antenatal infection. An intravenous (i.v.) route of administration was selected to avoid species differences in gastrointestinal uptake and metabolism associated with oral administration. We also assessed amniotic-to-fetal transfer following i.a. administration for comparison with our previous studies of azithromycin and erythromycin biodistribution using the same model (26).
MATERIALS AND METHODS
Surgical procedures and antibiotic administration.
All experimental procedures described in this study were approved by the Animal Ethics Committee of The University of Western Australia. Details of animal management, anesthesia, surgical catheterization, and recovery have been described previously (26). Five days after surgery, at 116 ± 1 days gestation, chronically catheterized pregnant ewes (∼65 kg) were randomly selected to receive either (i) a single maternal i.v. infusion of 650 mg solithromycin (10 mg/kg maternal weight; n = 6 ewes) over 60 min in 325 ml infusion buffer (5.77 g/liter tartaric acid, 50 g/liter mannitol, 5 g/liter thioglycerol, buffered to pH 4.2 with NaOH), (ii) a single i.a. injection of 3.5 mg solithromycin (1.4 mg/kg fetal weight) in 3 ml perfusion buffer (n = 6 ewes), or (iii) a combination of maternal i.v. infusion and i.a. injection (n = 7 ewes). At this gestational age, fetal weight was estimated to be 2.5 kg, and amniotic fluid volume was approximately 250 ml. Maternal and fetal arterial blood and amniotic fluid (two sets of 1 ml) samples were collected into heparinized tubes 30 min before and immediately prior to the administration of the macrolide antibiotics as described above; after the completion of antibiotic administration, samples were taken at 0.5, 1, 2, 4, 8, 12, 24, 48, and 72 h. Fetal arterial PO2 (PaO2), PCO2 (PaCO2), O2 saturation (SaO2), pH (pHa), and electrolytes were measured with a Rapid Lab 1265 blood gas analyzer (Siemens, Germany). Fetal and maternal plasma and amniotic fluid samples were stored at −80°C until they were shipped frozen to MicroConstants, Inc. (San Diego, CA), for analysis of solithromycin and its metabolites.
Solithromycin concentration determination.
Concentrations of solithromycin and its two side-chain metabolites (CEM-214 and N-acetyl [NAc]–CEM-101) in ovine plasma (maternal and fetal) and amniotic fluid were assayed using high-performance liquid chromatography(HPLC)-tandem quadrupole mass spectrometry (MicroConstants' analytical method MN12084). K3EDTA (anticoagulant) and internal standards were added to the plasma and amniotic fluid samples, which were then diluted with water, extracted using solid-phase extraction well plates, and analyzed by reverse-phase HPLC using a Phenomenex Luna CN 100-Å column maintained at 25°C. The mobile phase was 35% solvent A (20.0 mM ammonium formate, 0.2% formic acid, 0.0002% citric acid in water) and 65% solvent B (0.1% formic acid in methanol-acetonitrile [50:50, vol/vol]) at a flow rate of 0.3 ml/min. The retention times of CEM-101, NAc–CEM-101, and CEM-214 were 2.1, 2.0, and 1.95 min, respectively. The mobile phase was nebulized using heated nitrogen in a Z spray source/interface set to electrospray positive ionization mode; the ionized compounds were detected using tandem MS (MS/MS). The calibration range of the assay was from 10 to 20,000 ng/ml for solithromycin and from 1 to 2,000 ng/ml for both CEM-214 and NAc–CEM-101. CEM-101–d3 (BioLink Life Sciences, Inc.) and NAc–CEM-101–d6 (Microconstants Inc.) were used as the internal standards. The peak heights of solithromycin, CEM-214, NAc–CEM-101, and the internal standards and subsequent calibration curves were acquired using MassLynx version 4.1 (Waters, Milford, MA). All samples were analyzed in a total of nine assay runs. The coefficient of variation (CV) of the mean plasma quality control (QC) values (low, medium, and high) for solithromycin, CEM-214, and NAc–CEM-101 ranged from 3.86 to 4.93%, 9.92 to 23.4%, and 3.59 to 10.4%, respectively; in AF, the CV of the QC values ranged from 4.54 to 6.91%, 5.43 to 10.1%, and 3.34 to 7.96%, respectively. Methodological accuracy for all three analytes was 6.7 to 10.0%.
Statistical and pharmacokinetic analysis.
Antibiotic concentration data from n = 6 or 7 animals per time point were grouped, and the mean, standard deviation, and standard error of the mean were calculated. Pharmacokinetic analysis was performed using PKSolver software (45). Maternal and fetal solithromycin pharmacokinetic data were fitted using a two-compartment model, whereas the i.a. solithromycin administration data and all metabolite data were best described by a noncompartmental model.
RESULTS
Maternal i.v. administration.
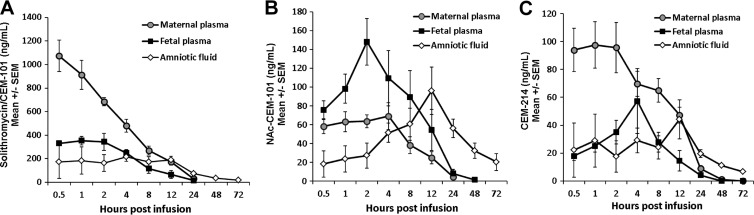
The pharmacokinetics of maternal i.v. solithromycin displayed characteristics in the pregnant sheep that are broadly similar to those reported in adult humans and other animal models (Fig. 1A). Peak concentrations of 1,073 ng/ml were obtained 30 min after maternal i.v. infusion (t = 1.5 h), which declined steadily with a half-life (t1/2) of 6 h, resulting in an area under the concentration-time curve from 0 to infinity (AUC0–∞) of greater than 6,500 ng/ml · h (Table 1). The acetylated metabolite NAc–CEM-101 was present in low but readily detectable levels in maternal plasma, reaching peak concentrations (69.2 ng/ml) at 4 h postinfusion that were approximately 7% of the maximal parent drug concentrations (Fig. 1B). The maternal plasma half-life of NAc–CEM-101 was similar to that of solithromycin, although the mean residence time (MRT) was slightly longer (8.5 h compared to 7.4 h) (Table 2). The bioactive metabolite CEM-214, which is formed by loss of the aminophenyl-1,2,3-triazole chain of solithromycin, was detected at levels higher than those of NAc–CEM-101 (97.6 ng/ml at 1 h postinfusion, 10.7% of the solithromycin concentration) and remained detectable for at least 24 h (Fig. 1C) with a t1/2 of 7.9 h (Table 3). At t = 2 h, the combined level of side-chain metabolites was 18% of the parent drug, somewhat higher than that reported for humans (approximately 8% after i.v. administration) (41).
FIG 1.
Concentrations of solithromycin (CEM-101) (A), N-acetyl solithromycin (NAc–CEM-101) (B), and CEM-214 (C) in maternal plasma, fetal plasma, and amniotic fluid after a single maternal dose of solithromycin (650 mg, 10 mg/kg maternal weight) by i.v. infusion. Data are shown as means ± standard errors of the means (SEM) (n = 6 or 7 animals).
TABLE 1.
Pharmacokinetic parameters of solithromycin in maternal, fetal, and amniotic compartments after administration of solithromycin via maternal i.v. infusion or i.a. injectiona
| Parameter | Value |
|||||
|---|---|---|---|---|---|---|
| Maternal i.v. |
i.a. injection |
|||||
| MP | FP | AF | MP | FP | AF | |
| Cmax (ng/ml) | 1,073.1 | 353.4 | 214.4 | 11.3 | 81.0 | 52,700 |
| Tmax (h) | 1.5 | 2 | 5 | 5 | 4 | 1.5 |
| t1/2 (h) | 6.0 | 6.2 | 21.5 | NC | 7.0 | 16.8 |
| AUC0–∞ (ng/ml · h) | 6,545 | 2,971 | 6,458 | NC | 981.5 | 317,808 |
| MRT0–∞ (h) | 7.4 | 8.5 | 30.6 | NC | 10.1 | 11.2 |
| CL (mg/ng/ml/h) | 0.099 | 0.22 | 0.101 | NC | 0.004 | <0.001 |
| Vss (mg/ng/ml) | 0.633 | 1.64 | 2.981 | NC | 0.033 | <0.001 |
MP, maternal plasma; FP, fetal plasma; AF, amniotic fluid. The start of infusion was designated as t = 0. AUC0–∞, area under the curve from 0 to infinity; MRT, mean residence time; CL, clearance; Vss, volume of distribution at steady state; Tmax, time to maximum concentration of drug in serum; NC, not calculated due to insufficient data; Cmax, maximum concentration of drug in serum.
TABLE 2.
Pharmacokinetic parameters of N-acetyl–CEM-101 in maternal, fetal, and amniotic compartments after administration of solithromycin via maternal i.v. infusion or i.a. injectiona
| Parameter | Value |
|||||
|---|---|---|---|---|---|---|
| Maternal i.v. |
i.a. injection |
|||||
| MP | FP | AF | MP | FP | AF | |
| Cmax (ng/ml) | 69.12 | 148.3 | 96.2 | 2.7 | 33.2 | 512.2 |
| Tmax (h) | 5 | 3 | 13 | 9 | 5 | 9 |
| t1/2 (h) | 6.0 | 7.6 | 33.8 | 12.2 | 6.7 | 21.8 |
| AUC0–∞ (ng/ml · h) | 789.5 | 1,635.7 | 4,226.5 | 64.2 | 490.8 | 18,414 |
| MRT0–∞ (h) | 8.5 | 9.7 | 49.2 | 19.1 | 11.5 | 33.0 |
| CL (mg/ng/ml/h) | 0.823 | 0.397 | 0.154 | 0.055 | 0.007 | 0.0002 |
| Vss (mg/ng/ml) | 6.16 | 3.47 | 7.41 | 0.99 | 0.075 | 0.0061 |
The start of infusion was designated as t = 0. MP, maternal plasma; FP, fetal plasma; AF, amniotic fluid; AUC0–∞, area under the curve from 0 to infinity; MRT, mean residence time; CL, clearance; Vss, volume of distribution at steady state; Tmax, time to maximum concentration of drug in serum; Cmax, maximum concentration of drug in serum.
TABLE 3.
Pharmacokinetic parameters of CEM-214 in maternal, fetal, and amniotic compartments after administration of solithromycin via maternal i.v. infusion or a combination of i.v. infusion and i.a. injectiona
| Parameter | Value |
|||||
|---|---|---|---|---|---|---|
| Maternal i.v. |
Combined i.v. and i.a. |
|||||
| MP | FP | AF | MP | FP | AF | |
| Cmax (ng/ml) | 9.6 | 57.3 | 44.2 | 125.8 | 48.3 | 198.2 |
| Tmax (h) | 2 | 5 | 13 | 2 | 9 | 25 |
| t1/2 (h) | 7.9 | 6.9 | 31.6 | 12.4 | 8.1 | 14.9 |
| AUC0–∞ (ng/ml · h) | 1,292 | 538 | 1,582 | 2,800 | 868 | 7,782 |
| MRT0–∞ (h) | 10.4 | 9.4 | 42.3 | 15.9 | 12.9 | 29.9 |
| CL (mg/ng/ml/h) | 0.503 | 1.21 | 0.411 | 0.232 | 0.748 | 0.084 |
| Vss (mg/ng/ml) | 4.74 | 10.2 | 16.95 | 3.46 | 8.93 | 2.41 |
Concentrations of CEM-214 with i.a. injection alone were low or nondetectable in all compartments and insufficient for pharmacokinetic calculations. The start of infusion was designated as t = 0. MP, maternal plasma; FP, fetal plasma; AF, amniotic fluid; AUC0–∞, area under the curve from 0 to infinity; MRT, mean residence time; CL, clearance; Vss, volume of distribution at steady state; Tmax, time to maximum concentration of drug in serum; Cmax, maximum concentration of drug in serum.
Solithromycin concentrations in fetal plasma peaked 1 to 2 h postinfusion, reaching concentrations of ∼350 ng/ml (Fig. 1A), representing a placental transfer efficiency of ∼34%. Clearance from the fetal compartment was similar to that in the maternal circulation, with a t1/2 of 6.2 h; fetal circulating concentrations were stable for the first 2 h after infusion and remained at therapeutic levels for over 12 h following a single maternal dose. The AUC0–∞ in the fetal compartment was 2,971 ng/ml · h (Table 1). In the fetal compartment, NAc–CEM-101 levels reached almost half those of CEM-101 at 2 h postinfusion (148 ng/ml); however, it was cleared more slowly, with a half-life of 7.6 h and an MRT of 9.7 h (Table 2) (Fig. 1B). Levels of CEM-214 were lower than NAc–CEM-101 (35 ng/ml at 2 h postinfusion) but exhibited a similar pharmacokinetic profile (Table 3).
Amniotic fluid concentrations of solithromycin had the lowest maximum concentration of drug in serum (Cmax) of the three compartments, 214 ng/ml at 4 to 8 h after maternal infusion; however, due to its very slow clearance and long half-life (21.5 h), therapeutic levels were sustained at >30 ng/ml for over 48 h (Fig. 1A). Accordingly, the AUC0–∞ in amniotic fluid was high: 6,458 ng/ml · h (Table 1). Concentrations of NAc–CEM-101 in AF rose steadily during the first 12 h postinfusion, reaching a Cmax of 96 ng/ml at 12 h, before declining slowly to 20 ng/ml at 72 h (Fig. 1B). This resulted in a long MRT (49 h) and a high AUC (4,226 ng/ml · h) (Table 2). CEM-214 also slowly accumulated in AF during the first 12 h postinfusion (Fig. 1C), exhibiting a t1/2 of 31.6 h and a Cmax at 13 h of 44.2 ng/ml (Table 3). Both metabolites were still detectable in AF at the 72-h time point.
Intra-amniotic administration.
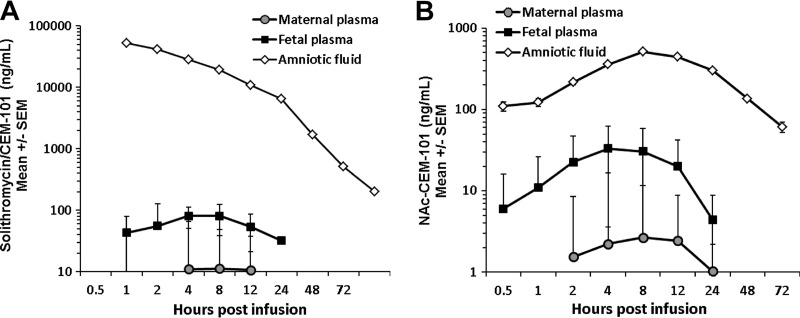
Administration of solithromycin (3.5 mg) into the amniotic cavity resulted in peak AF concentrations ranging from 18.9 to 92.8 μg/ml (mean, 52.7 μg/ml) (Fig. 2A). Concentrations declined slowly but steadily, with a t1/2 of >16 h; the AUC0–∞ was extremely high at 317,000 ng/ml · h, and concentrations remained well above therapeutic levels (>100 ng/ml) throughout the 72-h experimental period (Fig. 2A). Transfer from the amniotic to fetal compartment was poor, however, with fetal plasma levels peaking at ∼1.5% of maximal amniotic fluid levels (81 ng/ml at 2 h postinfusion). Nevertheless, fetal plasma solithromycin levels were sustained above 30 ng/ml for 24 h (t1/2 of 7 h; MRT of >10 h) (Fig. 2A). Maternal concentrations barely exceeded the limit of quantitation (10 ng/ml) and only at the 4- to 12-h time points, indicating a very low efficiency of AF-to-maternal plasma transfer (<0.05%).
FIG 2.
Concentrations of solithromycin (A) and its metabolite NAc–CEM-101 (B) in maternal plasma, fetal plasma, and amniotic fluid after a single amniotic dose (3.5 mg, 1.4 mg/kg fetal weight) by intra-amniotic (i.a.) injection. Data are shown as means ± SEM (n = 6 or 7 animals). Note log concentration axes. CEM-214 concentrations were at or below the limit of detection (data not shown).
NAc–CEM-101 was readily detectable after i.a. solithromycin administration in all three compartments, peaking at 8 h in amniotic fluid at levels nearly 5% of those of the parent drug at the same time point (512 ng/ml) (Fig. 2B). The MRT in amniotic fluid (33 h) was considerably longer than that for the parent drug (11 h). Fetal concentrations were lower (Cmax of 33 ng/ml at 4 h postinfusion) but showed a similar profile, falling after t = 9 h postinfusion and remaining detectable until after 24 h. Maternal levels also peaked at 9 h but never exceeded 3 ng/ml. The levels of CEM-214 in all three compartments following i.a. solithromycin administration were on the borderline of detectability and data could not be analyzed.
Combined maternal and intra-amniotic administration.
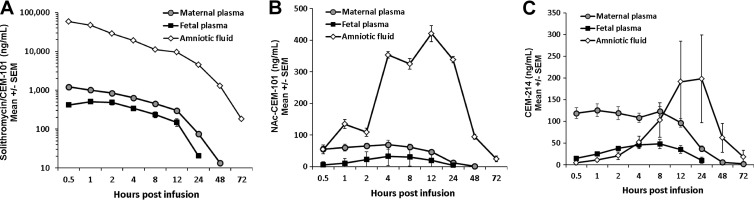
The combination of maternal i.v. and i.a. administration resulted in peak fetal concentrations about 25% higher than levels that would be predicted on a simple additive basis (Fig. 3A). At 1 h postinjection/postinfusion, fetal plasma levels reached 511 ng/ml and then declined with a t1/2 of 5.2 h; the AUC0–∞ was 4,880 ng/ml · h (Table 1). In the amniotic cavity, the combination of i.v. plus i.a. administration did not initially alter CEM-101 concentrations above the expected levels, but between 12 to 28 h the levels were sustained at >2-fold-higher concentrations than the simple combination would have predicted (Fig. 3A). Maternal concentrations were similar to those expected.
FIG 3.
Concentrations of solithromycin (CEM-101) (A), NAc–CEM-101 (B), and CEM-214 (C) in maternal plasma, fetal plasma, and amniotic fluid after combined maternal (10 mg/kg) and intra-amniotic (1.4 mg/kg) administration of solithromycin. Data are shown as means ± SEM (n = 6 or 7 animals). Note the log concentration axis in panel A.
Levels of NAc–CEM-101 in all three compartments were within the range expected based on a simple combination of the i.v. and i.a. doses (Fig. 3B). However, CEM-214 concentrations in AF showed an interesting and unexpected response to the combination dose (Fig. 3C and Table 3). With combined i.v. and i.a. CEM-101 administration, AF levels of CEM-214 showed a marked late-onset rise, reaching ∼200 ng/ml at 8 to 24 h postinfusion. This is 4-fold higher than that observed in the i.v.-only group, despite the fact that in the i.a. group, CEM-214 concentrations in AF were undetectable. The AF AUC0–∞ in the combined i.v. and i.a. group was much higher than in the maternal i.v. group (7,782 compared to 1,582 ng/ml · h). Plasma CEM-214 levels were predictable and did not show this counterintuitive response to the combined dose.
Comparison with azithromycin.
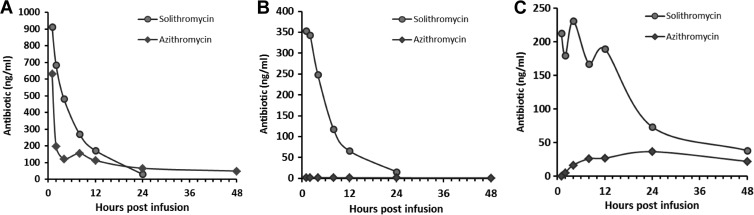
Our previous studies of macrolide biodistribution in pregnancy using the same model at the same gestational age observed very low rates of transfer of macrolides from the maternal compartment to the fetal or amniotic compartment (26). To highlight the differences between solithromycin and azithromycin, maternal plasma, fetal plasma, and AF concentrations of both antibiotics were plotted on the same graph for 48 h after maternal i.v. infusion. As shown in Fig. 4, the concentrations of solithromycin in the fetal and amniotic compartments were dramatically higher than azithromycin, with solithromycin achieving and sustaining therapeutic concentrations for at least 12 h (48 h in the amniotic cavity). At the 4-h time point, the maternal-to-fetal plasma and maternal-to-AF plasma transfer rates of azithromycin were 1.4% and 13.3%, respectively, whereas the equivalent figures for solithromycin were 51.6% and 47.9%.
FIG 4.
Comparison of solithromycin and azithromycin pharmacokinetic profiles in maternal plasma (A), fetal plasma (B), and amniotic fluid (C) after a single maternal dose by i.v. infusion (10 mg/kg solithromycin, 5 mg/kg azithromycin; single dose).
DISCUSSION
The central finding of this study is that a single dose of solithromycin given maternally can be an effective mode of delivery of antibiotic to both the fetal and amniotic compartments. Assuming this finding is replicated in human pregnancy, this is an extremely significant observation clinically, as currently available macrolides have limited oral bioavailability and do not transfer efficiently from the maternal compartment to the fetal compartment (unless given over extended periods of time). Consequently, maternal therapy provides poor antimicrobial protection for the amniotic cavity (including the fetus). This is likely to be a major factor contributing to the poor success rates of macrolides in the prevention of preterm birth and reduction of associated neonatal morbidity and mortality. The fact that solithromycin is considerably more potent than existing macrolides, has a high level of oral bioavailability, exhibits significant cellular accumulation, and, most importantly, is very effective against most macrolide-resistant strains of target organisms (31) makes maternal solithromycin administration an extremely attractive therapeutic prospect for treating and preventing intrauterine infections in pregnancy.
In adult humans, solithromycin pharmacokinetic parameters (Cmax, t1/2, and AUC0–∞) following a 600-mg oral dose are 862 ng/ml, 5.5 h, and 9,049 ng/ml · h, respectively (42). These data are reasonably similar to the present figures in maternal sheep plasma at a similar dose (1,073 ng/ml, 6.0 h, 6,545 ng/ml · h, respectively), suggesting that the pharmacokinetics are broadly similar in both species. While the ovine placenta differs structurally and anatomically from the human placenta in a number of important ways (46), the pregnant ewe remains a useful model to study placental transport and fetal growth and development (47). In our earlier studies in the same model, azithromycin (at half the dose) was cleared from maternal plasma considerably more rapidly than solithromycin (t1/2 of ∼1.3 h), although the AUC0–∞ was similar (6,227 ng/ml · h) (26). Surprisingly, in light of the immaturity of the fetus in terms of drug metabolic and secretory capacity, the t1/2 and MRT of solithromycin in fetal plasma appear to be only slightly longer than in the maternal circulation. This may relate to the mode of excretion of solithromycin. In adults, there is some evidence to suggest that solithromycin and an N-demethylated metabolite are eliminated in the bile (Cempra Inc., unpublished findings). Both human and ovine fetuses are known to have an immature but partially functional biliary secretion system (48, 49), presumably capable of eliminating small amounts of solithromycin. Repeated doses, therefore, may be expected to result in delayed elimination and associated changes in pharmacokinetic parameters. Future studies will need to explore this to ensure that fetal concentrations do not increase to toxic levels following multiple doses.
Fetal plasma solithromycin concentrations reached peak levels (∼350 to 400 ng/ml) 1 h after maternal infusion, suggesting a relatively high rate of transplacental passage. The efficiency of transfer (30 to 50%) was much greater than existing macrolides, which are <1% in the same ovine model (26) and <4% in humans (25). The relatively static levels of solithromycin found in the amniotic fluid probably reflects the initial accumulation of the antibiotic from fetal excretion and transplacental and transchorioamnion diffusion, in conjunction with a low rate of clearance (t1/2 of ∼20 h, similar to the t1/2 of azithromycin in AF of 17.5 h [26]). Hence, despite the Cmax in AF barely exceeding 200 ng/ml, the AUC was equivalent to that in MP (>6,000 ng/ml · h) and more than twice that in FP (2,971 ng/ml · h). Collectively, these data suggest that a single maternal 650-mg solithromycin dose is sufficient to give adequate therapeutic antibiotic levels in the fetal and amniotic compartments.
The MIC90 for solithromycin against most macrolide-susceptible strains of Ureaplasma spp., Mycoplasma spp., streptococci, and staphylococci is <30 ng/ml (31, 34, 38). Farrell and colleagues tested solithromycin in vitro against a large number (>10,000) of clinical bacterial pathogens and reported that the large majority of isolates of Streptococcus pneumoniae, Staphylococcus aureus, coagulase-negative staphylococci, beta-hemolytic streptococci, viridans group streptococci, and Moraxella catarrhalis were inhibited by 60 ng/ml solithromycin (34). Based on this cutoff, a single maternal dose would be expected to provide antimicrobial coverage in maternal plasma, fetal plasma, and AF for >12, >12, and >24 h, respectively. More resistant strains of these organisms and of other less susceptible species, such as Haemophilus influenzae and enterococci, required higher concentrations (500 to 2,000 ng/ml) to achieve effective inhibition (34). Repeated maternal administration is likely to be needed to achieve these concentrations in the amniotic cavity. Alternatively, as we have shown in this study, a single intra-amniotic dose would be sufficient in theory to eliminate even highly resistant microorganisms from the amniotic cavity. A combined i.v.-i.a. regimen might, therefore, have significant therapeutic advantages in terms of eradication of resistant or persistent intra-amniotic infections unresponsive to maternal administration alone.
Detailed studies of solithromycin metabolism and the bioactivity of its metabolites have not yet been carried out. In preliminary studies, the two known urinary excretion products of solithromycin metabolism, NAc–CEM-101 and CEM-214, have been found to possess approximately 50% and 25% of the activity of the parent compound against susceptible strains of Gram-positive organisms, although against resistant organisms they are markedly less effective (41). Their relative efficacy against Ureaplasma and Mycoplasma spp. is currently being evaluated, although these studies are not yet complete; therefore, definitive assessment of their bioactivity is currently lacking. These metabolites were both present in the maternal circulation at low but readily detectable levels, 5 to 10% of the concentrations of the parent drug. In this regard, the sheep and mouse are similar. Humans, on the other hand, produce less of both metabolites, while monkeys produce ∼10-fold more and rats produce more NAc–CEM-101 but almost no CEM-214 after oral administration (41). The delayed clearance of the metabolites in AF results in extended duration of their effects (i.e., high AUC) in this compartment. Taking into account the preliminary data available on the relative potency of CEM-101 and its metabolites (approximately 1:0.5:0.25) and applying this to the individual AUC values for CEM-101 and its metabolites, the net combined AUCs in maternal plasma, fetal plasma, and AF following the maternal i.v. dose are 7,263, 3,923, and 8,967 ng/ml · h, respectively. Hence, the metabolites are likely to play a particularly significant contribution to solithromycin's antimicrobial effects in the amniotic cavity.
Solithromycin's spectrum of activity is particularly pertinent to the treatment of bacterial genital tract infections. Not only is it extremely effective against Mollicutes (Ureaplasma and Mycoplasma spp.), the organisms most commonly isolated from amniotic fluid following preterm labor or PPROM, but it is also highly effective against other important genital tract pathogens, such as group B streptococci, N. gonorrhoeae, and C. trachomatis (32, 34–40). Assuming that the present findings can be replicated in human pregnancy, then administration of solithromycin to women in preterm labor or PPROM would be expected to constitute a markedly more effective antenatal antimicrobial therapy than currently used macrolides, with commensurate benefits in the reduction of neonatal infections and associated morbidity and mortality. For asymptomatic women early in pregnancy at risk of infection-driven preterm birth, maternal solithromycin administration may offer an effective therapeutic approach for the prevention of preterm birth. Studies are under way to assess the transfer and metabolism of solithromycin by the human placenta.
ACKNOWLEDGMENTS
This work was supported by the National Health and Medical Research Council of Australia, Women and Infants Research Foundation, Western Australia, Channel 7 Telethon Trust, Western Australia, and Siemens Australia, Cempra Inc., NC, USA.
The assistance of the staff of The University of Western Australia's Large Animal Facility and our commercial sheep suppliers, Sara and Andrew Ritchie of Icon Agriculture, Darkan, Western Australia, are gratefully acknowledged.
Footnotes
Published ahead of print 4 November 2013
REFERENCES
- 1.Goldenberg RL, Hauth JC, Andrews WW. 2000. Intrauterine infection and preterm delivery. N. Engl. J. Med. 342:1500–1507. 10.1056/NEJM200005183422007 [DOI] [PubMed] [Google Scholar]
- 2.Romero R, Gotsch F, Pineles B, Kusanovic JP. 2007. Inflammation in pregnancy: its roles in reproductive physiology, obstetrical complications, and fetal injury. Nutr. Rev. 65:S194–S202. 10.1111/j.1753-4887.2007.tb00362.x [DOI] [PubMed] [Google Scholar]
- 3.Kim SM, Romero R, Lee J, Mi Lee S, Park CW, Shin Park J, Yoon BH. 2012. The frequency and clinical significance of intra-amniotic inflammation in women with preterm uterine contractility but without cervical change: do the diagnostic criteria for preterm labor need to be changed? J. Matern. Fetal Neonat. Med. 25:1212–1221. 10.3109/14767058.2011.629256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim MJ, Romero R, Gervasi MT, Kim JS, Yoo W, Lee DC, Mittal P, Erez O, Kusanovic JP, Hassan SS, Kim CJ. 2009. Widespread microbial invasion of the chorioamniotic membranes is a consequence and not a cause of intra-amniotic infection. Lab. Invest. 89:924–936. 10.1038/labinvest.2009.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee SM, Lee KA, Kim SM, Park CW, Yoon BH. 2011. The risk of intra-amniotic infection, inflammation and histologic chorioamnionitis in term pregnant women with intact membranes and labor. Placenta 32:516–521. 10.1016/j.placenta.2011.03.012 [DOI] [PubMed] [Google Scholar]
- 6.Keelan JA. 2011. Pharmacological inhibition of inflammatory pathways for the prevention of preterm birth. J. Reprod. Immunol. 88:176–184. 10.1016/j.jri.2010.11.003 [DOI] [PubMed] [Google Scholar]
- 7.DiGiulio DB, Gervasi M, Romero R, Mazaki-Tovi S, Vaisbuch E, Kusanovic JP, Seok KS, Gomez R, Mittal P, Gotsch F, Chaiworapongsa T, Oyarzun E, Kim CJ, Relman DA. 2010. Microbial invasion of the amniotic cavity in preeclampsia as assessed by cultivation and sequence-based methods. J. Perinat. Med. 38:503–513. 10.1515/JPM.2010.078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Han YW, Shen T, Chung P, Buhimschi IA, Buhimschi CS. 2009. Uncultivated bacteria as etiologic agents of intra-amniotic inflammation leading to preterm birth. J. Clin. Microbiol. 47:38–47. 10.1128/JCM.01206-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kacerovsky M, Pliskova L, Bolehovska R, Skogstrand K, Hougaard DM, Tsiartas P, Jacobsson B. 2012. The impact of the microbial load of genital mycoplasmas and gestational age on the intensity of intraamniotic inflammation. Am. J. Obstet. Gynecol.206:342e1–342e8. 10.1016/j.ajog.2012.01.004 [DOI] [PubMed] [Google Scholar]
- 10.Novy MJ, Duffy L, Axthelm MK, Sadowsky DW, Witkin SS, Gravett MG, Cassell GH, Waites KB. 2009. Ureaplasma parvum or Mycoplasma hominis as sole pathogens cause chorioamnionitis, preterm delivery, and fetal pneumonia in rhesus macaques. Reprod. Sci. 16:56–70. 10.1177/1933719108325508 [DOI] [PubMed] [Google Scholar]
- 11.Oh KJ, Lee KA, Sohn YK, Park CW, Hong JS, Romero R, Yoon BH. 2010. Intraamniotic infection with genital mycoplasmas exhibits a more intense inflammatory response than intraamniotic infection with other microorganisms in patients with preterm premature rupture of membranes. Am. J. Obstet. Gynecol. 203:211e1–211e8. 10.1016/j.ajog.2010.03.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DiGiulio DB. 2012. Diversity of microbes in amniotic fluid. Semin. Fetal Neonatal Med. 17:2–11. 10.1016/j.siny.2011.10.001 [DOI] [PubMed] [Google Scholar]
- 13.Subramaniam A, Abramovici A, Andrews WW, Tita AT. 2012. Antimicrobials for preterm birth prevention: an overview. Infect. Dis. Obstet. Gynecol. 2012:157159. 10.1155/2012/157159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brocklehurst P, Gordon A, Heatley E, Milan SJ. 2013. Antibiotics for treating bacterial vaginosis in pregnancy. Cochrane Database Syst. Rev. 1:CD000262. 10.1002/14651858.CD000262.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mercer B. 2012. Antibiotics in the management of PROM and preterm labor. Obstet. Gynecol. Clin. North Am. 39:65–76. 10.1016/j.ogc.2011.12.007 [DOI] [PubMed] [Google Scholar]
- 16.Barros FC, Bhutta ZA, Batra M, Hansen TN, Victora CG, Rubens CE, GAPPS Review Group 2010. Global report on preterm birth and stillbirth (3 of 7): evidence for effectiveness of interventions. BMC Pregnancy Childbirth 10(Suppl 1):S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lamont RF, Nhan-Chang CL, Sobel JD, Workowski K, Conde-Agudelo A, Romero R. 2011. Treatment of abnormal vaginal flora in early pregnancy with clindamycin for the prevention of spontaneous preterm birth: a systematic review and metaanalysis. Am. J. Obstet. Gynecol. 205:177–190. 10.1016/j.ajog.2011.03.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sarkar M, Woodland C, Koren G, Einarson AR. 2006. Pregnancy outcome following gestational exposure to azithromycin. BMC Pregnancy Childbirth 6:18. 10.1186/1471-2393-6-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morency AM, Bujold E. 2007. The effect of second-trimester antibiotic therapy on the rate of preterm birth. J. Obstet. Gynaecol. Can. 29:35–44 [DOI] [PubMed] [Google Scholar]
- 20.Mylonas I. 2010. Antibiotic chemotherapy during pregnancy and lactation period: aspects for consideration. Arch. Gynecol. Obstet. 283:7–18. 10.1007/s00404-010-1646-3 [DOI] [PubMed] [Google Scholar]
- 21.Bahat Dinur A, Koren G, Matok I, Wiznitzer A, Uziel E, Gorodischer R, Levy A. 2013. The fetal safety of macrolides. Antimicrob. Agents Chemother. 57:3307–3311. 10.1128/AAC.01691-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kenyon S, Boulvain M, Neilson J. 2001. Antibiotics for preterm premature rupture of membranes. Cochrane Database Syst. Rev. 4:CD001058. 10.1002/14651858.CD001058 [DOI] [PubMed] [Google Scholar]
- 23.McDonald H, Brocklehurst P, Parsons J. 2005. Antibiotics for treating bacterial vaginosis in pregnancy. Cochrane Database Syst. Rev. 1:CD000262. 10.1002/14651858.CD000262.pub3 [DOI] [PubMed] [Google Scholar]
- 24.Dando SJ, Nitsos I, Newnham JP, Jobe AH, Moss TJ, Knox CL. 2010. Maternal administration of erythromycin fails to eradicate intrauterine ureaplasma infection in an ovine model. Biol. Reprod. 83:616–622. 10.1095/biolreprod.110.084954 [DOI] [PubMed] [Google Scholar]
- 25.Heikkinen T, Laine K, Neuvonen PJ, Ekblad U. 2000. The transplacental transfer of the macrolide antibiotics erythromycin, roxithromycin and azithromycin. Br. J. Obstet. Gynacol. 107:770–775. 10.1111/j.1471-0528.2000.tb13339.x [DOI] [PubMed] [Google Scholar]
- 26.Keelan JA, Nitsos I, Saito M, Musk GC, Kemp MW, Timmins M, Li S, Yaegashi N, Newnham JP. 2011. Maternal-amniotic-fetal distribution of macrolide antibiotics following intravenous, intramuscular, and intraamniotic administration in late pregnant sheep. Am. J. Obstet. Gynecol. 204:546e10–7. 10.1016/j.ajog.2011.02.035 [DOI] [PubMed] [Google Scholar]
- 27.Kiefer L, Rubin A, McCoy J, Foltz EL. 1955. The placental transfer of erythomycin. Am. J. Obstet. Gynecol. 69:174–177 [DOI] [PubMed] [Google Scholar]
- 28.Philipson A, Sabath LD, Charles D. 1973. Transplacental passage of erythromycin and clindamycin. N. Engl. J. Med. 288:1219–1221. 10.1056/NEJM197306072882307 [DOI] [PubMed] [Google Scholar]
- 29.Ramsey PS, Vaules MB, Vasdev GM, Andrews WW, Ramin KD. 2003. Maternal and transplacental pharmacokinetics of azithromycin. Am. J. Obstet. Gynecol. 188:714–718. 10.1067/mob.2003.141 [DOI] [PubMed] [Google Scholar]
- 30.Grigsby PL, Novy MJ, Sadowsky DW, Morgan TK, Long M, Acosta E, Duffy LB, Waites KB. 2012. Maternal azithromycin therapy for ureaplasma intraamniotic infection delays preterm delivery and reduces fetal lung injury in a primate model. Am. J. Obstet. Gynecol. 207:475e1–475e14. 10.1016/j.ajog.2012.10.871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Waites KB, Crabb DM, Duffy LB. 2009. Comparative in vitro susceptibilities of human mycoplasmas and ureaplasmas to a new investigational ketolide, CEM-101. Antimicrob. Agents Chemother. 53:2139–2141. 10.1128/AAC.00090-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Putnam SD, Castanheira M, Moet GJ, Farrell DJ, Jones RN. 2010. CEM-101, a novel fluoroketolide: antimicrobial activity against a diverse collection of Gram-positive and Gram-negative bacteria. Diagn. Microbiol. Infect. Dis. 66:393–401. 10.1016/j.diagmicrobio.2009.10.013 [DOI] [PubMed] [Google Scholar]
- 33.Roblin PM, Kohlhoff SA, Parker C, Hammerschlag MR. 2010. In vitro activity of CEM-101, a new fluoroketolide antibiotic, against Chlamydia trachomatis and Chlamydia (Chlamydophila) pneumoniae. Antimicrob. Agents Chemother. 54:1358–1359. 10.1128/AAC.01343-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Farrell DJ, Castanheira M, Sader HS, Jones RN. 2010. The in vitro evaluation of solithromycin (CEM-101) against pathogens isolated in the United States and Europe (2009). J. Infect. 61:476–483. 10.1016/j.jinf.2010.08.010 [DOI] [PubMed] [Google Scholar]
- 35.Golparian D, Fernandes P, Ohnishi M, Jensen JS, Unemo M. 2012. In vitro activity of the new fluoroketolide solithromycin (CEM-101) against a large collection of clinical Neisseria gonorrhoeae isolates and international reference strains, including those with high-level antimicrobial resistance: potential treatment option for gonorrhea? Antimicrob. Agents Chemother. 56:2739–2742. 10.1128/AAC.00036-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Putnam SD, Sader HS, Farrell DJ, Biedenbach DJ, Castanheira M. 2011. Antimicrobial characterisation of solithromycin (CEM-101), a novel fluoroketolide: activity against staphylococci and enterococci. Int. J. Antimicrob. Agents 37:39–45. 10.1016/j.ijantimicag.2010.08.021 [DOI] [PubMed] [Google Scholar]
- 37.Rodgers W, Frazier AD, Champney WS. 2013. Solithromycin inhibition of protein synthesis and ribosome biogenesis in Staphylococcus aureus, Streptococcus pneumoniae, and Haemophilus influenzae. Antimicrob. Agents Chemother. 57:1632–1637. 10.1128/AAC.02316-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Llano-Sotelo B, Dunkle J, Klepacki D, Zhang W, Fernandes P, Cate JH, Mankin AS. 2010. Binding and action of CEM-101, a new fluoroketolide antibiotic that inhibits protein synthesis. Antimicrob. Agents Chemother. 54:4961–4970. 10.1128/AAC.00860-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lemaire S, Van Bambeke F, Tulkens PM. 2009. Cellular accumulation and pharmacodynamic evaluation of the intracellular activity of CEM-101, a novel fluoroketolide, against Staphylococcus aureus, Listeria monocytogenes, and Legionella pneumophila in human THP-1 macrophages. Antimicrob. Agents Chemother. 53:3734–3743. 10.1128/AAC.00203-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Woosley LN, Castanheira M, Jones RN. 2010. CEM-101 activity against Gram-positive organisms. Antimicrob. Agents Chemother. 54:2182–2187. 10.1128/AAC.01662-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pereira DE, Degenhardt TP, Fernandes P. 2010. Comparison of CEM-101 metabolism in mice, rats, monkeys and humans. Abstr. 50th Intersci. Conf. Antimicrob. Agents Chemother. (ICAAC), Boston, MA American Society for Microbiology, Washington, DC [Google Scholar]
- 42.Still JG, Schranz J, Degenhardt TP, Scott D, Fernandes P, Gutierrez MJ, Clark K. 2011. Pharmacokinetics of solithromycin (CEM-101) after single or multiple oral doses and effects of food on single-dose bioavailability in healthy adult subjects. Antimicrob. Agents Chemother. 55:1997–2003. 10.1128/AAC.01429-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oldach D, Clark K, Schranz J, Das A, Craft JC, Scott D, Jamieson BD, Fernandes P. 2013. Randomized, double-blind, multi-center, phase 2 study comparing the efficacy and safety of oral solithromycin (CEM-101) to oral levofloxacin in the treatment of patients with community-acquired bacterial pneumonia. Antimicrob. Agents Chemother. 57:2526–2534. 10.1128/AAC.00197-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bertrand D, Bertrand S, Neveu E, Fernandes P. 2010. Molecular characterization of off-target activities of telithromycin: a potential role for nicotinic acetylcholine receptors. Antimicrob. Agents Chemother. 54:5399–5402. 10.1128/AAC.00840-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang Y, Huo M, Zhou J, Xie S. 2010. PKSolver: an add-in program for pharmacokinetic and pharmacodynamic data analysis in Microsoft Excel. Comput. Methods Programs Biomed. 99:306–314. 10.1016/j.cmpb.2010.01.007 [DOI] [PubMed] [Google Scholar]
- 46.Carter AM. 2007. Animal models of human placentation—a review. Placenta 28(Suppl A):S41–S47 [DOI] [PubMed] [Google Scholar]
- 47.Newnham JP, Kallapur SG, Kramer BW, Moss TJ, Nitsos I, Ikegami M, Jobe AH. 2003. Betamethasone effects on chorioamnionitis induced by intra-amniotic endotoxin in sheep. Am. J. Obstet. Gynecol. 189:1458–1466. 10.1067/S0002-9378(03)00758-0 [DOI] [PubMed] [Google Scholar]
- 48.Macias RI, Marin JJ, Serrano MA. 2009. Excretion of biliary compounds during intrauterine life. World J. Gastroenterol. 15:817–828. 10.3748/wjg.15.817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ring JA, Ghabrial H, Ching MS, Potocnik S, Shulkes A, Smallwood RA, Morgan DJ. 1994. Hepatic uptake and excretion of [14C]sodium taurocholate by the isolated perfused fetal sheep liver. Biochem. Pharmacol. 48:667–674. 10.1016/0006-2952(94)90043-4 [DOI] [PubMed] [Google Scholar]