LETTER
Neisseria meningitidis can cause potentially fatal systemic disease. Early diagnosis and prompt antimicrobial intervention are critical for favorable clinical outcomes. Antibiotic resistance has been reported for penicillins (1), tetracycline (2), and sulfonamides (3), as well as quinolones (4) and rifampin (5).
N. meningitidis expresses two major porins, PorA and PorB, which are antigenically variable between strains and within a strain, and PorA is phase variable (random on/off switching) (6). Neisseria gonorrhoeae expresses a single porin, PorB. Changes in porin expression or variant porins mediate antibiotic resistance in several Gram-negative bacteria, including N. gonorrhoeae (7–10). In N. meningitidis, the absence of PorB increases resistance to tetracycline and cefsulodin in vitro (11). The role of PorA in antimicrobial resistance has not been reported for the meningococcus. In addition to its proposed role in immune evasion, we hypothesized that phase-variable PorA expression may provide an obvious mechanism for the meningococcus to evade antimicrobials if PorA mediates antibiotic uptake or exclusion. We generated strains lacking PorA or PorB and conducted MIC assays. We also tested whether altered PorA expression is selected by antimicrobial exposure during the course of the MIC assay.
The porA and porB genes with flanking sequences were amplified from N. meningitidis strain MC58 and cloned into pGEM T-easy. Inverse PCR followed by self-ligation yielded plasmids with internal deletions and introduced restriction sites. The LacZ/kanamycin cassette (12) was cloned into the introduced SmaI site of the deleted PorA allele, yielding plasmid pPorALacZKan. A chloramphenicol acetyltransferase gene was amplified and cloned into the introduced BglII site of the deleted porB allele, in plasmid pPorB:CAT. The porA lacZ kan or porB::cat constructs were transformed into N. meningitidis strain ¢9 (13) to yield strains ¢9ΔPorA and ¢9ΔPorB. Allelic replacement of wild-type porA or porB alleles with the mutant allele was confirmed by PCR and sequencing, as well as SDS-PAGE of Sarkosyl-extracted outer membrane proteins (Fig. 1A).
FIG 1.
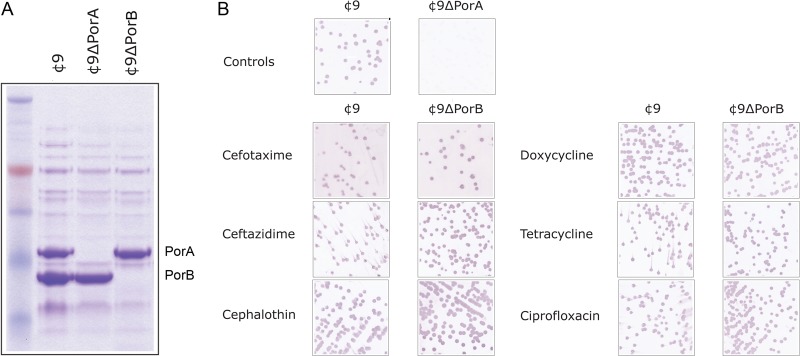
Analysis of porin expression during MIC analysis. (A) Membrane proteins were isolated by Sarkosyl extraction, and 10 μg was separated on 8 to 12% bis-Tris acrylamide gels prior to Coomassie staining. Lane 1, ¢9; lane 2, ¢9ΔPorA; lane 3, ¢9ΔPorB. (B) Samples from the last well showing turbidity were plated on BHI agar and immunoblotted with the PorA-specific MAb MN14C11.6.
MIC were assessed by broth microdilution method in 96-well plates (14) using bacteria grown overnight on supplemented BHI agar at 37°C and subcultured shaking for approximately 4 h in BHI broth at 37°C before adjusting to approximately 5 × 105 CFU/ml based on the optical density at 600 nm (OD600). After addition of 50 μl to serially diluted antibiotics, MICs were recorded after overnight growth at 37°C (Table 1) as the concentrations at which no turbidity was observed. Each assay was done three times, each time in triplicate. For each treatment, MICs were identical within and between assays.
TABLE 1.
MICs for the wild-type and mutant N. meningitidis strainsa
| Antibiotic | MIC (μg/ml) |
||
|---|---|---|---|
| ¢9 | ¢9ΔPorA | ¢9ΔPorB | |
| Cefotaxime | 0.003125 | 0.003125 | 0.00625 |
| Ceftazidime | 0.03125 | 0.03125 | 0.0625 |
| Cephalothin | 0.3125 | 0.3125 | 0.625 |
| Ampicillin | 0.0625 | 0.0625 | 0.0625 |
| Carbenicillin | 0.0625 | 0.0625 | 0.0625 |
| Cloxacillin | 1.25 | 1.25 | 1.25 |
| Penicillin G | 0.03125 | 0.03125 | 0.03125 |
| Piperacillin | 0.03125 | 0.03125 | 0.015625 |
| Tetracycline | 0.3125 | 0.3125 | 0.625 |
| Doxycycline | 0.1875 | 0.1875 | 0.375 |
| Ciprofloxacin | 0.003125 | 0.003125 | 0.00625 |
| Nalidixic acid | 1.25 | 1.25 | 1.25 |
| Imipenem | 0.0625 | 0.0625 | 0.0625 |
| Rifampin | 0.125 | 0.125 | 0.0625 |
MICs are reported as the last well in which turbidity was observed. Bold indicates reduced susceptibility of ¢9ΔPorB.
Our results confirmed that loss of meningococcal PorB expression increases resistance to tetracycline (11). Mutations in PorB also contribute to resistance to tetracycline in N. gonorrhoeae (9, 15). Recommended combination therapy for multiply resistant N. gonorrhoeae includes injectable ceftriaxone and oral doxycycline or azithromycin (16). In this context, it is notable that the meningococcal ¢9ΔPorB mutant strain had reduced susceptibility to doxycycline. We noted decreased susceptibility to cephalothin for ¢9ΔPorB and also for the cephalosporins cefotaxime and ceftazidime. A previous report linked N. meningitidis PorB mutation with increased cefsulodin resistance (11). In N. gonorrhoeae, PorB loop 3 variants also contribute to enhanced cephalosporin resistance (17). Our confirmation that Neisseria PorB modulates cephalosporin susceptibility raises the possibility that reduction in susceptibility to this class may arise clinically or be facilitated by mutations in PorB in either meningococci or gonococci.
Fluoroquinolone use is no longer recommended for gonococcal infection (18), and point mutations in PorB1b of N. gonorrhoeae contribute to decreased susceptibility of N. gonorrhoeae to ciprofloxacin. Expression changes in gonococcal porin alter ciprofloxacin resistance (19); we found that mutation of meningococcal PorB also results in reduced ciprofloxacin susceptibility. Conversely, this strain was more susceptible to rifampin, the other major choice for meningococcal prophylaxis, perhaps through altered membrane architecture, as has been suggested for altered rifamycin resistance of colistin-resistant Acinetobacter baumannii (20).
In contrast, we observed no changes in susceptibility of ¢9ΔPorA compared to the PorA+ parent strain for any of the antibiotics tested (Table 1). This suggests that either PorA has no direct role in entry of antimicrobials into the cell or there is selection for reduced PorA expression via phase variation during the MIC assay, either in the parental strain or in ¢9ΔPorB, potentially masking PorA-mediated antibiotic entry. To assess this, we isolated bacteria from the final well in which turbidity was observed and assessed PorA expression by colony immunoblotting using the anti-P1.7 murine monoclonal antibody (MAb) MN14C11.6 (obtained from NIBSC, United Kingdom). As controls, reactivity was compared between the parental strain ¢9 and the PorA mutant strain ¢9ΔPorA. This showed that PorA expression was unaltered between control wells and after exposure to any of the tested antimicrobials (see Fig. 1B); thus, decreased susceptibility of ¢9ΔPorB is not due to altered PorA expression levels.
Our results are consistent with previous reports for a role for N. meningitidis PorB in cephalosporins and tetracycline resistance (9, 11, 15). The parallels with gonococcal PorB1b with respect to antibiotic resistance are striking. Knocking out PorB expression also decreased meningococcal susceptibility to doxycycline, one of the recommended therapies for multiply resistant N. gonorrhoeae. Troublingly, this suggests that selective pressure may lead to emergence of gonococcal PorB variants with reduced doxycycline susceptibility. Although we recorded only 2-fold differences that are unlikely in isolation to lead to treatment failures, the synergy of PorB variation with other mutations has the potential to expand the meningococcal and gonococcal resistance spectrum. We found no evidence of a role for PorA in antimicrobial transit across the outer membrane.
ACKNOWLEDGMENT
NHMRC program grant 565526 supports M.P.J.
Footnotes
Published ahead of print 21 October 2013
REFERENCES
- 1.Bertrand S, Carion F, Wintjens R, Mathys V, Vanhoof R. 2012. Evolutionary changes in antimicrobial resistance of invasive Neisseria meningitidis isolates in Belgium from 2000 to 2010: increasing prevalence of penicillin nonsusceptibility. Antimicrob. Agents Chemother. 56:2268–2272. 10.1128/AAC.06310-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crawford SA, Fiebelkorn KR, Patterson JE, Jorgensen JH. 2005. International clone of Neisseria meningitidis serogroup A with tetracycline resistance due to tet(B). Antimicrob. Agents Chemother. 49:1198–1200. 10.1128/AAC.49.3.1198-1200.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fiebelkorn KR, Crawford SA, Jorgensen JH. 2005. Mutations in folP associated with elevated sulfonamide MICs for Neisseria meningitidis clinical from five continents. Antimicrob. Agents Chemother. 49:536–540. 10.1128/AAC.49.2.536-540.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Castanheira M, Deshpande LM, Jones RN, Farrell DJ. 2012. Evaluation of quinolone resistance-determining region mutations and efflux pump expression in Neisseria meningitidis resistant to fluoroquinolones. Diagn. Microbiol. Infect. Dis. 72:263–266. 10.1016/j.diagmicrobio.2011.12.001 [DOI] [PubMed] [Google Scholar]
- 5.Carter PE, Abadi FJ, Yakubu DE, Pennington TH. 1994. Molecular characterization of rifampin-resistant Neisseria meningitidis. Antimicrob. Agents Chemother. 38:1256–1261. 10.1128/AAC.38.6.1256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van der Ende A, Hopman CT, Dankert J. 2000. Multiple mechanisms of phase variation of PorA in Neisseria meningitidis. Infect. Immun. 68:6685–6690. 10.1128/IAI.68.12.6685-6690.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Curtis NA, Eisenstadt RL, Turner KA, White AJ. 1985. Porin-mediated cephalosporin resistance in Escherichia coli K-12. J. Antimicrob. Chemother. 15:642–644. 10.1093/jac/15.5.642 [DOI] [PubMed] [Google Scholar]
- 8.De E, Basle A, Jaquinod M, Saint N, Mallea M, Molle G, Pages JM. 2001. A new mechanism of antibiotic resistance in Enterobacteriaceae induced by a structural modification of the major porin. Mol. Microbiol. 41:189–198. 10.1046/j.1365-2958.2001.02501.x [DOI] [PubMed] [Google Scholar]
- 9.Gill MJ, Simjee S, Al-Hattawi K, Robertson BD, Easmon CS, Ison CA. 1998. Gonococcal resistance to beta-lactams and tetracycline involves mutation in loop 3 of the porin encoded at the penB locus. Antimicrob. Agents Chemother. 42:2799–2803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pages JM, James CE, Winterhalter M. 2008. The porin and the permeating antibiotic: a selective diffusion barrier in Gram-negative bacteria. Nat. Rev. Microbiol. 6:893–903. 10.1038/nrmicro1994 [DOI] [PubMed] [Google Scholar]
- 11.Tommassen J, Vermeij P, Struyve M, Benz R, Poolman JT. 1990. Isolation of Neisseria meningitidis mutants deficient in class 1 (porA) and class 3 (porB) outer membrane proteins. Infect. Immun. 58:1355–1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Srikhanta YN, Maguire TL, Stacey KJ, Grimmond SM, Jennings MP. 2005. The phasevarion: a genetic system controlling coordinated, random switching of expression of multiple genes. Proc. Natl. Acad. Sci. U. S. A. 102:5547–5551. 10.1073/pnas.0501169102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Virji M, Makepeace K, Peak IR, Ferguson DJ, Jennings MP, Moxon ER. 1995. Opc- and pilus-dependent interactions of meningococci with human endothelial cells: molecular mechanisms and modulation by surface polysaccharides. Mol. Microbiol. 18:741–754. 10.1111/j.1365-2958.1995.mmi_18040741.x [DOI] [PubMed] [Google Scholar]
- 14.Clinical Laboratory Standards Institute 2009. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. M07-A8 Clinical Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 15.Olesky M, Zhao SQ, Rosenberg RL, Nicholas RA. 2006. Porin-mediated antibiotic resistance in Neisseria gonorrhoeae: ion, solute, and antibiotic permeation through PIB proteins with penB mutations. J. Bacteriol. 188:2300–2308. 10.1128/JB.188.7.2300-2308.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention 2012. Update to CDC's sexually transmitted diseases treatment guidelines, 2010: oral cephalosporins no longer a recommended treatment for gonococcal infections. MMWR Morb. Mortal. Wkly. Rep. 61:590–594 http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6131a3.htm [PubMed] [Google Scholar]
- 17.Lindberg R, Fredlund H, Nicholas R, Unemo M. 2007. Neisseria gonorrhoeae isolates with reduced susceptibility to cefixime and ceftriaxone: association with genetic polymorphisms in penA, mtrR, porB1b, and ponA. Antimicrob. Agents Chemother. 51:2117–2122. 10.1128/AAC.01604-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Newman LM, Moran JS, Workowski KA. 2007. Update on the management of gonorrhea in adults in the United States. Clin. Infect. Dis. 443:S84–101. 10.1086/511422 [DOI] [PubMed] [Google Scholar]
- 19.Lindback E, Islam S, Unemo M, Lang C, Wretlind B. 2006. Transformation of ciprofloxacin-resistant Neisseria gonorrhoeae gyrA, parE and porB1b genes. Int. J. Antimicrob. Agents 28:206–211. 10.1016/j.ijantimicag.2006.04.003 [DOI] [PubMed] [Google Scholar]
- 20.Li J, Nation RL, Owen RJ, Wong S, Spelman D, Franklin C. 2007. Antibiograms of multidrug-resistant clinical Acinetobacter baumannii: promising therapeutic options for treatment of infection with colistin-resistant strains. Clin. Infect. Dis. 45:594–598. 10.1086/520658 [DOI] [PubMed] [Google Scholar]


