Abstract
Daptomycin use at our institution changed to ideal body weight dosing based on a published analysis of pharmacokinetic-pharmacodynamic efficacy target attainment, bacterial ecology, and a desire to reduce drug toxicity. The current study compared outcomes between actual body weight and ideal body weight dosing of daptomycin before and after this intervention. In the evaluable group, 69 patients received doses based on actual body weight and 48 patients received doses based on ideal body weight. Patients were treated for documented Enterococcus species, Staphylococcus aureus, or coagulase-negative Staphylococcus infections, including bloodstream, intraabdominal, skin and soft tissue, urinary, and bone. There was no statistically significant difference in clinical success between the groups (88.9% for actual body weight compared to 89.1% for ideal body weight, P = 0.97). After we adjusted for gender, age, body mass index, concomitant 3-hydroxy-3-methylglutaryl–coenzyme A reductase inhibitors, infection type, and organism type, clinical success rates remained similar between groups (adjusted odds ratio of 0.68 in favor of actual body weight, 95% confidence interval [CI] of 0.13 to 3.55). Microbiological outcomes, length of stay, mortality, and adverse effects were also similar between groups. Further studies are warranted to confirm that ideal body weight dosing provides similar outcomes to actual body weight dosing for all patients and types of infections and organisms.
INTRODUCTION
Conventional daptomycin doses, based on actual body weight (ABW), result in sufficient drug exposure to meet an effective area under the curve (AUC)-to-MIC ratio. Dvorchik and Damphousse demonstrated that a single dose of 4 mg of daptomycin/kg of body weight administered to nonobese patients resulted in an AUC of 418 ± 25 μg · h/ml (1). A 6-mg/kg dose resulted in AUC values of 726 ± 79 μg · h/ml in healthy, nonobese patients (2). In a mouse thigh infection model, Safdar et al. determined that the 24-h AUC/MIC parameters associated with bacteriostatic effect were 388 to 537 for four ATCC Staphylococcus aureus strains and 0.94 to 1.67 for two clinical isolates of Enterococcus faecium (3).
Pharmacokinetic parameters may change in some patient populations, including the obese population. In two studies evaluating ABW-dosed daptomycin pharmacokinetics in the morbidly obese (1, 4), the AUC increased 30 to 60% compared to in normal-weight individuals. Volume of distribution (V) and clearance (CL) were reduced when normalized for weight.
Daptomycin dosing can be limited by toxicity, especially myositis and creatine phosphokinase (CPK) elevations with exposures exceeding 2 weeks of duration (5). In an examination of data from a randomized trial of daptomycin treatment of bacteremia and endocarditis, a direct relationship between minimum serum concentration (Cmin) and incidence of CPK elevation was established. Patients weighing ≥111 kg had a significantly higher probability of elevated Cmin than patients weighing <111 kg. Using a Monte Carlo evaluation, dosing daptomycin based on ideal body weight (IBW) in simulated patients weighing ≥111 kg predicted AUC values that were not significantly different from those obtained with ABW in simulated patients weighing <111 kg. More significantly, IBW in obese patients was predicted to reduce the risk of elevated Cmin concentrations and hence reduce toxicity in this model.
An internal review of all S. aureus and Enterococcus organisms at University of Wisconsin Hospital and Clinics collected between 2009 and 2010 was conducted and found that 98.9% of S. aureus and Enterococcus species were susceptible (MIC of ≤1 μg/ml or ≤4 μg/ml, respectively) by the Etest method to daptomycin.
Based on a highly susceptible bacterial ecology, two pharmacokinetic-pharmacodynamic (PK/PD) investigations suggesting that minimum efficacy parameters are attainable in all patients, and a potential to reduce the risk of drug toxicity in obese patients, our institution adopted daptomycin therapeutic dosing based on IBW in July 2010. The aim of this study was to describe and compare clinical and microbiological outcomes between ABW and IBW dosing.
MATERIALS AND METHODS
Patients who received daptomycin at the University of Wisconsin Hospital and Clinics between 1 July 2009 and 1 July 2011 were included in this investigation. Patients were older than 17 years, had a positive culture, and received daptomycin therapy for at least 72 h. Patients with endocarditis or prosthetic-device-related infections, without device removal, were excluded due to the prolonged treatment course and primary need for surgical management. Other exclusion criteria included ABW less than IBW, receipt of daptomycin at an outside institution within 24 h of admission, causative isolates known to be nonsusceptible to daptomycin, renal impairment, and preexisting rhabdomyolysis. Renal impairment was defined as a creatinine clearance less than 30 ml/min (calculated using the Cockcroft-Gault or Salazar-Corcoran equation for patients with ABW greater than 30% over IBW), daptomycin dosed every 48 h, or renal replacement therapy.
This retrospective analysis was approved by the institutional review board at our institution.
The primary outcome measure was clinical success. Clinical success was defined as the number of patients with clinical cure or improvement divided by the overall number of evaluable patients (6). Clinical cure was characterized as clinical signs and symptoms resolved and/or no additional antibiotics with Gram-positive coverage necessary. Clinical improvement included patients who required additional antibiotic therapy after daptomycin was stopped but whose condition allowed for deescalation of therapy. Clinical failure was defined as resistant, worsening, or new/recurrent clinical signs and symptoms of infection or the need for a change in antibiotic therapy (i.e., to a different antibiotic with similar coverage or an increase in dosage of daptomycin) (6).
Assessment of clinical cure, improvement, or failure was completed by a blinded physician and pharmacist panel which reviewed the patient cases but was unaware of daptomycin dosing weight cohort. The panel was provided patient demographic information, type of infection, culture results, antimicrobials used before, during, and/or after daptomycin therapy, duration of daptomycin therapy, and patient outcome if known. If the assessment was discordant, a third panelist assessed the outcome. If there was discordance among the previous three panelists, a fourth panelist assessed the outcome. If panelists judged that a patient could not be fairly categorized as cure, improvement, or failure, the patient was classified as nonevaluable for clinical outcome assessment. Other efficacy outcomes included microbiological success, hospital length of stay, and in-hospital mortality.
Microbiologic success was defined as the number of patients having at least one microbiologic result with microbiological cure (documented or presumed eradication) and no results with microbiological failure (documented or presumed failure) divided by the overall number of evaluable patients. Documented eradication was described as a subsequent negative culture that demonstrated elimination of the causative organism. Presumed eradication was used to classify those cases when no repeat cultures were obtained but the patient clinically improved and received no subsequent targeted therapy. Documented microbiological failure was described as failure to eradicate the original causative organism as documented by subsequent positive cultures. Presumed microbiological failure was used to classify those cases when no repeat cultures were obtained in the setting of clinical deterioration. Patients with no documented microbiological outcome and who were also nonevaluable for clinical outcome were deemed nonevaluable for microbiological outcome.
Incidence of CPK elevation, myopathy, and rhabdomyolysis were retrospectively reviewed to assess safety. The criteria for CPK elevation were adapted from the definitions used in the study by Bhavnani et al. to allow for assessment of patients who did not have baseline CPK values drawn (5). The normal reference range for CPK at UWHC is 0 to 175 U/liter. Patients were considered to have a CPK elevation if one of the following was true: no CPK elevations at baseline or no CPK checked at baseline followed by CPK elevations greater than three times the upper limit of normal (ULN), baseline CPK greater than the ULN followed by CPK elevations greater than five times the ULN, or daptomycin stopped by the treating physician due to concern for CPK elevation even if the degree of elevation did not meet the aforementioned criteria. If a patient had CPK elevation, the chart was reviewed for patient-reported symptoms of myopathy.
Wilcoxon rank-sum tests were used to compare continuous variables between patient groups. Chi-square tests were conducted to compare clinical and microbiological success rates, in-hospital mortality, and other categorical variables. Logistic regression was also used to compare clinical success, clinical cure, and microbiological success between groups, including terms adjusting for potentially confounding baseline covariates. Covariates identified through clinical considerations included age, gender, body mass index (BMI), organism type (a term for each Enterococcus species, methicillin-resistant S. aureus (MRSA), methicillin-susceptible S. aureus (MSSA), or coagulase-negative Staphylococcus infections), and infection type (a single term distinguishing bacteremia or osteomyelitis from intraabdominal, skin and soft tissue, or urinary tract). Concomitant 3-hydroxy-3-methylglutaryl–coenzyme A (HMG-CoA) reductase inhibitor use was significantly different between groups and so was also included. A sensitivity analysis was performed to assess the effect of model selection on results. Odds ratios (and adjusted odds ratios from logistic regression) were used to quantify group comparisons of success and cure rates. P values of <0.05 were considered statistically significant.
RESULTS
Patients.
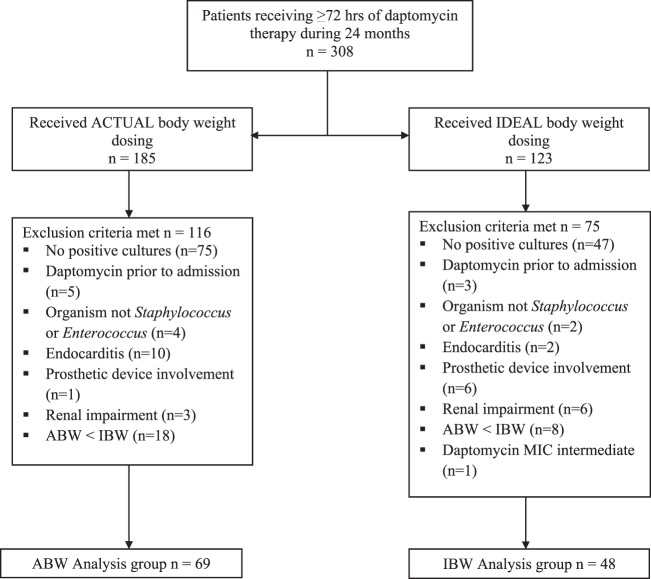
A total of 308 patients (n = 185 in the ABW group, n = 123 in the IBW group) received at least 72 h of daptomycin during the study period. The number of patients and reasons for exclusion are presented in Fig. 1. The main reason for exclusion was lack of positive cultures. Sixty-nine ABW patients and 48 IBW patients were available for clinical and microbiologic analysis. Six patients (8.7%) in the ABW group and two patients (4.2%) in the IBW group were judged to be clinically nonevaluable, and they were not included in the clinical outcome assessment. Nonevaluable classification reasons included inadequate information due to loss of follow-up, concomitant double Gram-positive antibiotic therapies, or infections that significantly confounded the outcome assessment. Four patients (5.8%) in the ABW group and one patient (2.1%) in the IBW group were nonevaluable for microbiological outcome and were not included in that assessment. Of the clinically nonevaluable patients, two patients (2.9%) in the ABW group and one patient (2.1%) in the IBW group had microbiological results and were included in the microbiological outcome assessment. For the above-described reasons, the numbers of patients included in the clinical and microbiological analyses were different.
FIG 1.
Patients evaluated for inclusion and reasons for exclusion.
Most baseline characteristics were similar between groups (Table 1). Use of HMG-CoA reductase inhibitors during daptomycin therapy was significantly higher in the ABW group than in the IBW group (49.3% compared to 22.9%, P < 0.01). More patients in the IBW group than in ABW group had enterococcal infections (75.0% compared to 56.5%, P = 0.04). Percentages of MRSA, MSSA, and coagulase-negative Staphylococcus were infrequent but similar. More patients in the IBW group than in the ABW group had urinary tract infections (25.0% compared to 10.1%, P = 0.03). There was a trend toward more osteomyelitis in the ABW group (10.1% compared to 2.1%, P = 0.09). Rates of bacteremia, skin and soft tissue infection, and intraabdominal infection were similar. Twenty-one patients (30.4%) in the ABW group and 18 patients (37.5%) in the IBW group had bacteremia.
TABLE 1.
Baseline characteristics between the ABW and IBW groups
| Characteristic | Result |
P value | |
|---|---|---|---|
| ABW (n = 69) | IBW (n = 48) | ||
| Mean age (yr) (SD) | 56.9 (13.6) | 57.6 (12.4) | 0.72 |
| Mean wt (kg) (SD)a | 91.0 (24.8) | 91.4 (21.9) | 0.61 |
| Mean ABW/IBW (%) (SD)a | 142 (44.0) | 141 (28.4) | 0.40 |
| Mean BMI (kg/m2) (SD)a | 30.9 (8.7) | 31.0 (6.2) | 0.44 |
| Mean serum creatinine (mg/dl) (SD) | 1.33 (0.66) | 1.35 (0.73) | 0.93 |
| No. (%) with BMI of >30 kg/m2 | 28 (40.6) | 25 (52.1) | 0.22 |
| No. (%) with general floor status | 57 (82.6) | 42 (87.5) | 0.47 |
| No. (%) male | 37 (53.6) | 28 (58.3) | 0.61 |
| No. (%) with medical condition | |||
| Diabetes | 26 (37.7) | 20 (41.7) | 0.66 |
| Cancer | 10 (14.5) | 12 (25.0) | 0.15 |
| Transplant | 22 (31.9) | 17 (35.4) | 0.69 |
| Surgery within past 30 days | 22 (31.9) | 10 (20.8) | 0.19 |
| No. (%) with concomitant HMG-CoA reductase inhibitors | 34 (49.3) | 11 (22.9) | <0.01 |
| No. (%) with infection typeb,c | |||
| Bacteremia | 21 (30.4) | 18 (37.5) | 0.43 |
| Skin and soft tissue | 26 (37.7) | 14 (29.2) | 0.34 |
| Osteomyelitis | 7 (10.1) | 1 (2.1) | 0.09 |
| Intraabdominal | 17 (24.6) | 15 (31.2) | 0.43 |
| Urinary tract | 7 (10.1) | 12 (25.0) | 0.03 |
| No. (%) with organism typec | |||
| Enterococcus | 39 (56.5) | 36 (75.0) | 0.04 |
| CoNS | 24 (34.8) | 13 (27.1) | 0.99 |
| MRSA | 13 (18.8) | 9 (18.8) | 0.23 |
| MSSA | 9 (13.0) | 3 (6.25) | 0.38 |
| No. (%) with prior antimicrobial therapy with activity against the isolated organism | 35 (50.7) | 19 (39.6) | 0.23 |
| No. (%) with concomitant antimicrobial therapy with activity against the isolated organism | 7 (10.3) | 8 (16.7) | 0.31 |
| Mean daily daptomycin dose (mg) (SD) | 441 (130) | 328 (84.1) | <0.01 |
| Daily daptomycin dosing rates | |||
| 4 mg/kg | 39 (56.5) | 27 (56.2) | 0.98 |
| 6 mg/kg | 30 (43.5) | 21 (43.8) | |
| Mean duration (days) of therapy (SD) | 21.8 (26.1) | 20.6 (19.5) | 0.89 |
| No. (%) who completed therapy as outpatient | 33 (48.5) | 27 (56.2) | 0.41 |
| Mean no. (SD) of inpatient daily doses of daptomycin | 8.4 (5.7) | 8.1 (7.5) | 0.28 |
| Mean no. (SD) of outpatient daily doses of daptomycin | 13.3 (24.6) | 13.1 (17.6) | 0.47 |
Excludes one outlying subject with a weight of 339.5 kg.
All infection types are off-label usage of daptomycin except bacteremia (S. aureus) and skin and soft tissue.
Some patients had more than one infection or organism type identified that was treated with daptomycin, but the number of patients was used as the denominator to calculate the percentage reported.
Excluding one IBW subject with an outlying weight of 339.5 kg, mean weights were similar at 91.0 kg (range, 49.3 to 185.0) and 91.4 kg (range, 52.0 to 150.0) in the ABW and IBW groups, respectively (P = 0.61), and ABW was 142% and 141% of IBW in the ABW and IBW dosing groups, respectively (P = 0.40). Nominal dosing rates (4 mg/kg compared to 6 mg/kg) were similar (P = 0.98), as was duration of therapy (P = 0.89). Actual daily dose was 441 ± 130 mg and 328 ± 84 mg in the ABW and IBW groups, respectively (P < 0.01). Excluding the subject with outlying weight, actual dosing in the IBW group was 135 ± 103 mg less than it would have been if ABW dosing had been used. On average, IBW group subjects received an actual dose of 73.8 ± 14.0% of their hypothetical ABW dose.
Outcomes.
The blinded panel that determined clinical outcomes was concordant for 75.2% of patients. Discordance was resolved with a third reviewer for 19.7% of patients. A fourth reviewer was needed to resolve the remainder (5.1%).
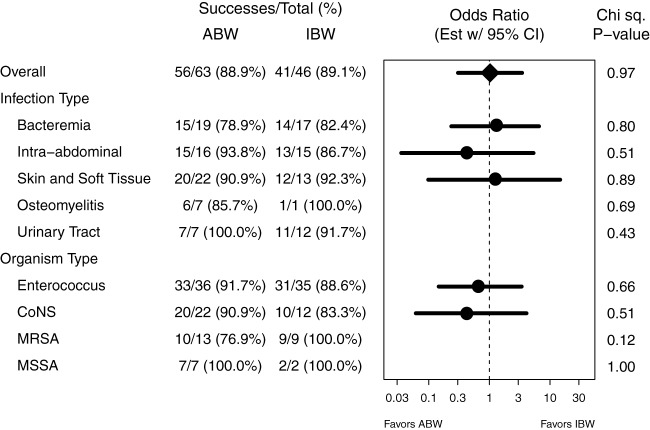
There were no statistically significant differences between overall clinical or microbiological outcomes between the ABW and IBW groups (Table 2, all P values were ≥0.19). The effect of ABW compared to IBW dosing on clinical success was similar overall and within subgroups defined by the presence of each type of infection and type of organism (Fig. 2, all P values were ≥0.12). In particular, for patients with MRSA, clinical success was similar between ABW and IBW groups (76.9% compared to 100%, P = 0.12). Similarly, there was no evidence that the effect of ABW compared to IBW dosing on clinical success differed by concomitant HMG-CoA reductase inhibitor use (P = 0.31) or body mass index (P = 0.44).
TABLE 2.
Comparison of outcomes between the ABW and IBW groups
| Outcome | Value |
P value | |
|---|---|---|---|
| ABW (n = 63) | IBW (n = 46) | ||
| No. (%) with clinical successa | 56/63 (88.9) | 41/46 (89.1) | 0.97 |
| No. (%) with clinical cure | 36/63 (57.1) | 32/46 (69.6) | 0.19 |
| No. (%) with microbiological successb | 59/65 (90.8) | 43/47 (91.5) | 0.48 |
| Mean length (days) of hospital stay (SD) | |||
| Overall | 20.8/68 (16.8) | 20.8/48 (22.2) | 0.34 |
| Since collection of first positive culture | 15.7/68 (14.1) | 16.3/48 (14.1) | 0.82 |
| Since start of daptomycin | 12.7/68 (12.4) | 12.8/48 (13.7) | 0.48 |
| No. (%) with in-hospital mortality | 5/68 (7.3) | 2/48 (4.2) | 0.49 |
| No. (%) with death related to infection | 1/68 (1.5) | 0/48 (0.0) | 0.40 |
Six patients in the ABW group and two patients in the IBW group were classified as clinically nonevaluable. Of these patients, two in the ABW group and one in the IBW group had microbiological results and were included in the microbiological analysis.
Four patients in the ABW group and one patient in the IBW group were classified as microbiologically nonevaluable.
FIG 2.
Clinical success rates by dosing group, overall and by infection type and organism type subgroups. Odds ratio estimates with 95% confidence intervals are shown for those subgroups with sufficient data. Chi-square tests of an association between clinical success and dosing group are given by subgroup.
After we adjusted for gender, age, BMI, concomitant HMG-CoA reductase inhibitors, infection type, and organism type, clinical cure rates were similar between the ABW and IBW groups (adjusted odds ratio of 2.10 for the IBW group, 95% confidence interval [CI] of 0.79 to 5.54, 80% CI of 1.11 to 3.16). Clinical success rates were also similar between the ABW and IBW groups (adjusted odds ratio of 0.68 for the ABW group, 95% CI of 0.13 to 3.55). A sensitivity analysis to assess the effect of model selection on results was performed for all subsets of the above-described covariates, and estimates and confidence intervals were insensitive to model choice.
A large number of patients did not have follow-up cultures documented (65% of the ABW group, 50% of the IBW group). Among patients with documented eradication or failure, there was no statistically significant difference in documented microbiological outcomes between groups (P = 1.00).
Length of hospital stay was similar between the ABW and IBW groups (Table 2). Duration of hospital stay from collection of first positive culture (15.7 ± 14.1 days compared to 16.3 ± 14.1 days, P = 0.82) and from start of daptomycin therapy (12.7 ± 12.4 compared to 12.8 ± 13.7 days, P = 0.48) did not differ between groups. Five patients (7.3%) in the ABW group and two patients (4.2%) in the IBW group died during the hospital stay (P = 0.49).
Safety.
There were no significant differences in the number of adverse events in either group. CPK monitoring was absent in 46 patients (29 in the ABW group, 17 in the IBW group, P = 0.60) during therapy. As a result, these patients were unable to be assessed for elevations. Eight ABW patients (11.6%) and five IBW patients (10.4%) experienced an adverse event. Of patients with CPK measurements, one patient in the ABW group (and zero patients in the IBW group) experienced a CPK elevation. In this patient, CPK values increased by two and a half times the ULN, which did not meet the previously defined criteria for an elevation; however, daptomycin therapy was discontinued by the medical team due to concerns regarding the CPK elevation. One patient in each group developed presumed daptomycin pulmonary toxicity. Six ABW patients (8.7%) and four IBW patients (8.3%) developed eosinophilia.
DISCUSSION
To our knowledge, this is the first study to report clinical outcomes of patients receiving daptomycin doses based on IBW. Conventional daptomycin dosing at 4 mg/kg or 6 mg/kg results in AUC values of 418 or 726 μg · h/ml in nonobese patients and significantly higher AUC values in obese patients, both of which exceed the minimum PK/PD parameters associated with efficacy, including S. aureus and Enterococcus species. Our results suggest that IBW dosing has similar clinical and microbiological success rates to those achieved with ABW dosing for some infections. Overall, the clinical and microbiologic success rates when using IBW dosing were 89.1% and 91.7%. These success rates are similar to a prior effectiveness study demonstrating a 93% clinical success rate for a variety of S. aureus infection types (6). Furthermore, there were no significant differences between hospital length of stay, in-hospital mortality, or incidence of adverse effects.
Daptomycin is often a target for antimicrobial stewardship programs because of the poor clinical outcomes associated with MRSA infection and the associated high costs of Staphylococcal infection and drug acquisition. Kullar et al. recently reported the use of daptomycin in a MRSA bacteremia treatment pathway for organisms with a high vancomycin MIC (7). They found that clinical success rates improved after a switch to high-dose daptomycin. The same group found that mortality and persistent bacteremia significantly decreased when daptomycin therapy was provided early in cases of MRSA bacteremia with a vancomycin MIC of >1 μg/ml (8). Fortunately at our institution, the incidence of a high vancomycin MIC (>1 μg/ml) represents only 7% of MRSA isolates (internal data). Three studies have evaluated the use of high-dose (>6 mg/kg/day) daptomycin in a variety of infection types, including endocarditis, bacteremia, osteomyelitis, skin and soft tissue, and urinary tract infection, with a variety of organisms, including S. aureus, coagulase-negative Staphylococcus, and/or enterococcal infections (9–11). High-dose daptomycin was found to be safe and effective in all reports.
The majority of patients in our study received daptomycin for the treatment of enterococcal infections. Only 34 of 117 (29.1%) patients received therapy for S. aureus infection; however, it does not appear that organism type had a significant effect on clinical outcomes. In particular, clinical success was statistically indistinguishable between ABW and IBW groups among patients with S. aureus, but the success rate was higher in the IBW group. When adjusting for baseline variables, including organism type, the odds ratio for clinical success favored ABW, while the odds ratio for clinical cure favored IBW, but in neither case was the difference statistically significant. Given the small overall sample size and the low numbers of patients with S. aureus or coagulase-negative Staphylococcus, it is difficult to completely exclude any difference in outcomes between ABW and IBW groups when considering specific organism type. In models adjusting for age, gender, BMI, HMG-CoA reductase inhibitors, infection type, and organism type, there was no statistically significant difference in outcomes between ABW and IBW groups, although both had large confidence intervals. With respect to clinical cure, our results can rule out an odds ratio of 0.9 or less (that is, ABW being 10% more effective or better than IBW) with 96% confidence (one sided). It is difficult to rule out superiority of ABW to IBW with respect to clinical success due to the large variance. However, given the favorable odds ratio for clinical cure in the IBW group and similar overall clinical success rates in both groups, it seems unlikely that IBW treatment led to significantly poorer patient outcomes.
Our data demonstrated no difference in clinical or microbiologic outcomes when adjusted for body mass index. This is consistent with prior studies which suggest that ABW dosing produces significantly higher AUC values in obese individuals than in normal-weight individuals and the idea that AUC values obtained with IBW dosing are adequate to meet minimum PK-PD parameters. Physiochemically, daptomycin has a small V (0.1 liters/kg) and is likely to remain serum concentrated with minimal tissue distribution (12). Dvorchik and Damphousse confirmed this finding and demonstrated that, in obesity, the V decreased when adjusted for body weight (1). Since this was a retrospective study, we were unable to collect blood samples for PK analysis and determination of AUC/MIC values. The numbers of adverse events in each group were small but similar. It is interesting to note that the rate of CPK elevation in our study was much lower than reported by Bhavnani et al. (5), which demonstrated an increased risk of this event with prolonged daptomycin duration. Our findings may be attributed to the fact that complex infections requiring prolonged antibiotic duration, including endocarditis and prosthetic-device-related infections, without device removal, were excluded from our study. Although HMG-CoA reductase inhibitors may increase the risk of CPK elevation and myopathy, patients who received these medications during daptomycin therapy did not have a higher rate of CPK elevations or clinical failures in our study. Therapy with these agents may also be a surrogate marker for unaccounted baseline characteristics and overall health status of patients who receive them. The statistically significant reduction in use of HMG-CoA reductase inhibitors between ABW and IBW groups was due to adoption of an institutional guideline protocolizing IBW dosing and recommending discontinuation of HMG-CoA reductase inhibitors during daptomycin therapy. We adjusted for this imbalance in our models to correct for direct effects and any correlated unaccounted baseline characteristics.
There are several limitations of our results. Labs, cultures, and other data used to determine outcome assessments were limited by what was available in the electronic medical records. As a result, follow-up cultures and CPK monitoring were not completed for all patients, and there was the potential for patients to be lost to follow-up. Our evaluation of adverse effects, specifically CPK, is limited. Over one-third of our patients did not have CPK documented during therapy, and the average duration of inpatient therapy, 8 days, is shorter than the typical time course for CPK elevation, myalgia, and rhabdomyolysis to begin to develop. Clinical and microbiological outcome definitions were subjective and relied upon medical record documentation; however, they were based on those used in a previous study. The analysis may be complicated by potential confounders, such as concomitant antimicrobials; however, similar percentages of patients in the IBW and ABW groups received antimicrobials with activity against the targeted organism. Given the small sample size, our study lacked the power to detect significant differences or superiority in outcomes or adverse events between ABW and IBW groups. Although our sample size was small, it is consistent with other dose finding studies where the sample sizes were small. Four prior studies that evaluated various daptomycin dosing regimens included 15, 24, 36, and 96 patients (2, 12–14). An extended-infusion piperacillin-tazobactam study included only 194 patients (15). There were 117 patients in our study, which is comparable to the sample sizes of other daptomycin and novel dosing strategy studies.
Dosing of daptomycin based on IBW may not be appropriate for all institutions for Staphylococcus infections. Empirical IBW dosing should be done cautiously and only after the incidence of organisms with high vancomycin MICs is evaluated and daptomycin MIC values are found to be highly susceptible. Targeted IBW dosing could be considered in this scenario. However, we have not evaluated this “step-down” method at our institution.
Conclusion.
Our study was the first to present clinical outcomes for IBW dosing. Our results suggest that ABW and IBW dosing may provide similar clinical and microbiological outcomes. Certain patient and infection characteristics may favor one dosing weight over another, and further study is warranted. Both infection and organism type did not appear to have a significant effect on outcomes in our study; however, more data and additional studies are needed to confirm that IBW dosing is comparable to ABW dosing for all infection types and organisms.
ACKNOWLEDGMENTS
The project described was supported by the Clinical and Translational Science Award (CTSA) program, through the NIH National Center for Advancing Translational Sciences (NCATS), grant UL1TR000427.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Published ahead of print 21 October 2013
REFERENCES
- 1.Dvorchik BH, Damphousse D. 2005. The pharmacokinetics of daptomycin in moderately obese, morbidly obese, and matched nonobese subjects. J. Clin. Pharmacol. 45:48–56. 10.1177/0091270004269562 [DOI] [PubMed] [Google Scholar]
- 2.Dvorchik BH, Brazier D, DeBruin MF, Arbeit RD. 2003. Daptomycin pharmacokinetics and safety following administration of escalating doses once daily to healthy subjects. Antimicrob. Agents Chemother. 47:1318–1323. 10.1128/AAC.47.4.1318-1323.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Safdar N, Andes D, Craig WA. 2004. In vivo pharmacodynamic activity of daptomycin. Antimicrob. Agents Chemother. 48:63–68. 10.1128/AAC.48.1.63-68.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pai MP, Norenberg JP, Anderson T, Goade DW, Rodvold KA, Telepak RA, Mercier RC. 2007. Influence of morbid obesity on the single-dose pharmacokinetics of daptomycin. Antimicrob. Agents Chemother. 51:2741–2747. 10.1128/AAC.00059-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhavnani SM, Rubino CM, Ambrose PG, Drusano GL. 2010. Daptomycin exposure and the probability of elevations in the creatine phosphokinase level: data from a randomized trial of patients with bacteremia and endocarditis. Clin. Infect. Dis. 50:1568–1574. 10.1086/652767 [DOI] [PubMed] [Google Scholar]
- 6.Sakoulas G, Brown J, Lamp KC, Friedrich LV, Lindfield KC. 2009. Clinical outcomes of patients receiving daptomycin for the treatment of Staphylococcus aureus infections and assessment of clinical factors for daptomycin failure: a retrospective cohort study utilizing the Cubicin Outcomes Registry and Experience. Clin. Ther. 31:1936–1945. 10.1016/j.clinthera.2009.09.012 [DOI] [PubMed] [Google Scholar]
- 7.Kullar R, Davis SL, Kaye KS, Levine DP, Pogue JM, Rybak MJ. 2013. Implementation of an antimicrobial stewardship pathway with daptomycin for optimal treatment of methicillin-resistant Staphylococcus aureus bacteremia. Pharmacotherapy 33:3–10. 10.1002/phar.1220 [DOI] [PubMed] [Google Scholar]
- 8.Murray KP, Zhao J, Davis SL, Kullar R, Kaye K, Lephart P, Rybak MJ. 2013. Early use of daptomycin versus vancomycin for methicillin-resistant Staphylococcus aureus bacteremia with vancomycin MIC >1 mg/liter: a matched cohort study. Clin. Infect. Dis. 56:1562–1569. 10.1093/cid/cit112 [DOI] [PubMed] [Google Scholar]
- 9.Moise PA, Hershberger E, Amodio-Groton MI, Lamp KC. 2009. Safety and clinical outcomes when utilizing high-dose (> or =8 mg/kg) daptomycin therapy. Ann. Pharmacother. 43:1211–1219. 10.1345/aph.1M085 [DOI] [PubMed] [Google Scholar]
- 10.Bassetti M, Nicco E, Ginocchio F, Ansaldi F, de Florentiis D, Viscoli C. 2010. High-dose daptomycin in documented Staphylococcus aureus infections. Int. J. Antimicrob. Agents 36:459–461. 10.1016/j.ijantimicag.2010.07.011 [DOI] [PubMed] [Google Scholar]
- 11.Kullar R, Davis SL, Levine DP, Zhao JJ, Crank CW, Segreti J, Sakoulas G, Cosgrove SE, Rybak MJ. 2011. High-dose daptomycin for treatment of complicated Gram-positive infections: a large, multicenter, retrospective study. Pharmacotherapy 31:527–536. 10.1592/phco.31.6.527 [DOI] [PubMed] [Google Scholar]
- 12.Woodworth JR, Nyhart EH, Jr, Brier GL, Wolny JD, Black HR. 1992. Single-dose pharmacokinetics and antibacterial activity of daptomycin, a new lipopeptide antibiotic, in healthy volunteers. Antimicrob. Agents Chemother. 36:318–325. 10.1128/AAC.36.2.318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benvenuto M, Benziger DP, Yankelev S, Vigliani G. 2006. Pharmacokinetics and tolerability of daptomycin at doses up to 12 milligrams per kilogram of body weight once daily in healthy volunteers. Antimicrob. Agents Chemother. 50:3245–3249. 10.1128/AAC.00247-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Katz DE, Lindfield KC, Steenbergen JN, Benziger DP, Blackerby KJ, Knapp AG, Martone WJ. 2008. A pilot study of high-dose short duration daptomycin for the treatment of patients with complicated skin and skin structure infections caused by Gram-positive bacteria. Int. J. Clin. Pract. 62:1455–1464. 10.1111/j.1742-1241.2008.01854.x [DOI] [PubMed] [Google Scholar]
- 15.Lodise TP, Jr, Lomaestro B, Drusano GL. 2007. Piperacillin-tazobactam for Pseudomonas aeruginosa infection: clinical implications of an extended-infusion dosing strategy. Clin. Infect. Dis. 44:357–363. 10.1086/510590 [DOI] [PubMed] [Google Scholar]