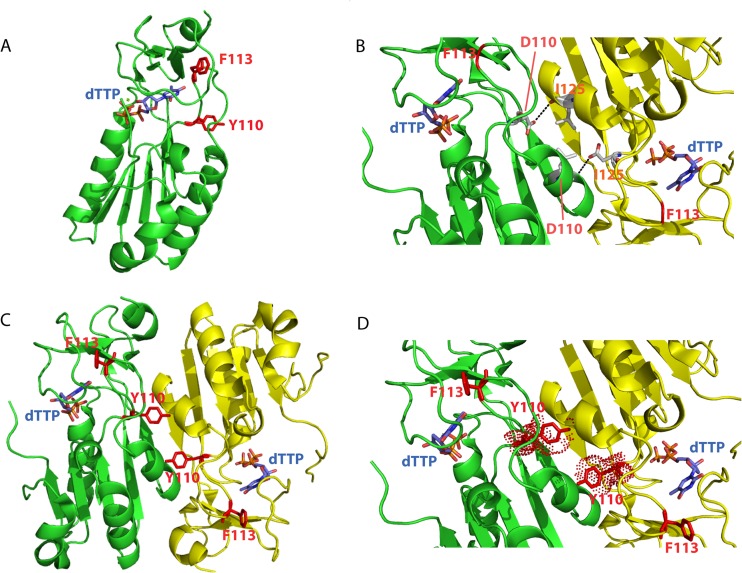
FIG 6.
Potential impact of D110Y mutation on substrate binding and thymidine kinase monomer assembly. (A) Tridimensional structure of one monomer of VACV TK in complex with dTTP (Protein Data Bank accession number 2J87). Residue F113, localized in the loop between the β5 sheet and α4 helix and required for substrate binding, is highlighted (see also Fig. 2). On the same loop is also shown the tyrosine residue (red sticks) found at position 110 and identified as the D110Y mutation. The presence of the mutated residue may disturb the loop and, as a consequence, substrate recognition. (B to D) Two monomers in complex with dTTP are shown in green and yellow. The phenylalanine at position 113 is depicted in red on each monomer. The aspartic acid at position 110 of one monomer establishes a direct interaction with the isoleucine 125 of the other monomer (B). Modification of the aspartic acid 110 into a tyrosine provokes a steric hindrance that may destabilize the assembly of monomers (C and D). The figures were generated using PyMol software (version 0.99; DeLano Scientific LLC).