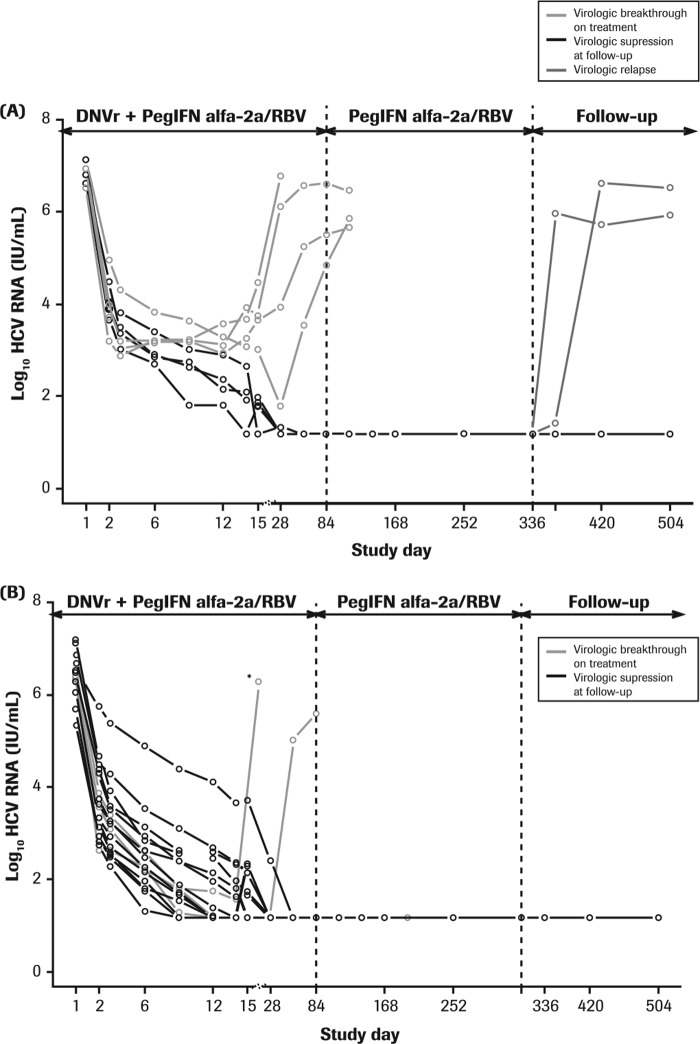
FIG 1.
Individual HCV RNA levels in G1a-infected (A) and G1b-infected (B) prior null responders during 12 weeks of treatment with DNVr plus PegIFN alfa-2a–RBV, followed by 36 weeks of PegIFN alfa-2a–RBV and the study follow-up period. DNVr, ritonavir-boosted danoprevir; G, genotype; PegIFN alfa-2a–RBV; peginterferon alfa-2a (40KD) at 180 μg/week plus 1,000 mg or 1,200 mg/day ribavirin. *, patient discontinued treatment at day 14 due to an adverse event.