TABLE 6.
Antibiotic resistance patterns of E. coli cultures tested
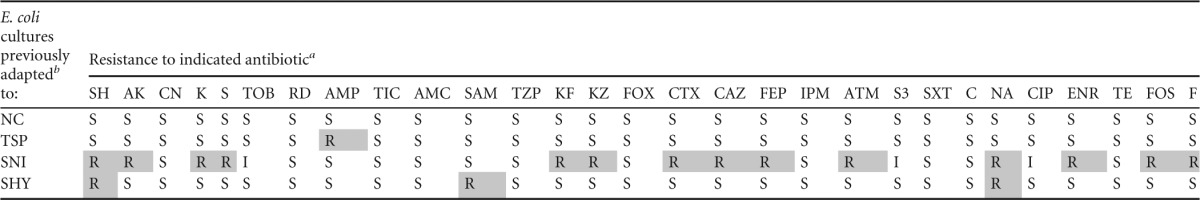
SH, spectinomycin (100 μg); AK, amikacin (30 μg); CN, gentamicin (10 μg); K, kanamycin (30 μg); S, streptomycin (10 μg); TOB, tobramycin (10 μg); RD, rifampin (5 μg); AMP, ampicillin (10 μg); TIC, ticarcillin (75 μg); AMC, amoxicillin-clavulanic acid (30 μg); SAM, ampicillin-sulbactam (20 μg); TZP, piperacillin-tazobactam (110 μg); KF, cephalothin (30 μg); KZ, cefazolin (30 μg); FOX, cefoxitin (30 μg); CTX, cefotaxime (30 μg); CAZ, ceftazidime (30 μg); FEP, cefepime (30 μg); IPM, imipenem (10 μg); ATM, aztreonam (30 μg); S3, sulfonamides (300 μg); SXT, trimethoprim-sulfamethoxazole (25 μg); C, chloramphenicol (30 μg); NA, nalidixic acid (30 μg); CIP, ciprofloxacin (5 μg); ENR, enrofloxacin (5 μg); TE, tetracycline (30 μg); FOS, phosphomycin (50 μg); F, nitrofurantoin (300 μg). Resistances are indicated as follows: R, resistant strain; I, strain of intermediate susceptibility; and S, susceptible strain. Data for exposed strains with increased resistance to antibiotics (relative to unexposed strains) are shaded; values for exposed strains not exhibiting increased susceptibility to antibiotics are not shaded. An increase in resistance was defined as a change from S (before exposure) to R (after exposure).
For adaptation, cultures were previously exposed to increasing subinhibitory concentrations of biocides. For additional information on interpretation, see the footnotes to Table 1.
