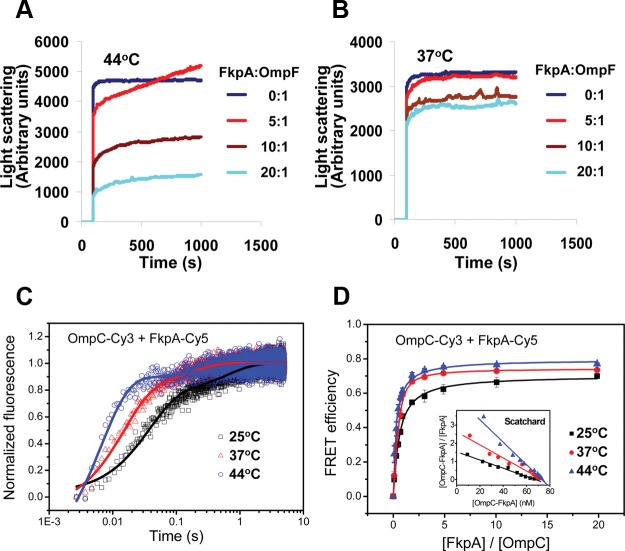
FIG 4.
Heat shock-induced activation of the in vitro chaperone activity of FkpA and its interaction with OMP. Shown is light scattering analysis of the chaperone-like activity of FkpA in preventing the aggregation of unfolded OmpF after dilution into PBS at 44°C (A) and 37°C (B). FkpA was present at 0, 2.5, 5, or 10 μM, and unfolded OmpF was diluted into a final concentration of 0.5 μM. (C) Fluorescence resonance energy transfer signals between Cy3-labeled FkpA and Cy5-labeled unfolded OmpC were observed on a stopped-flow instrument at 25°C, 37°C, and 44°C, with their relaxation times shown in Table 2. (D) Binding affinity between Cy3-labeled unfolded OmpC and Cy5-labeled FkpA. The acceptor Cy5 fluorescence intensities at 25°C, 37°C, and 44°C were plotted against the molar ratio of FkpA-Cy5 to OmpC-Cy3. Scatchard plots are shown in the inset. The Kd values obtained from the regression analysis are listed in Table 2.