Abstract
We report here the identification of waa clusters with the genes required for the biosynthesis of the core lipopolysaccharides (LPS) of two Plesiomonas shigelloides strains. Both P. shigelloides waa clusters shared all of the genes besides the ones flanking waaL. In both strains, all of the genes were found in the waa gene cluster, although one common core biosynthetic gene (wapG) was found in a different chromosome location outside the cluster. Since P. shigelloides and Klebsiella pneumoniae share a core LPS carbohydrate backbone extending up at least to the second outer-core residue, the functions of the common P. shigelloides genes were elucidated by genetic complementation studies using well-defined K. pneumoniae mutants. The function of strain-specific inner- or outer-core genes was identified by using as a surrogate acceptor LPS from three well-defined K. pneumoniae core LPS mutants. Using this strategy, we were able to assign a proteomic function to all of the P. shigelloides waa genes identified in the two strains encoding six new glycosyltransferases (WapA, -B, -C, -D, -F, and -G). P. shigelloides demonstrated an important variety of core LPS structures, despite being a single species of the genus, as well as high homologous recombination in housekeeping genes.
INTRODUCTION
Plesiomonas shigelloides is a species of rod-shaped Gram-negative bacteria recently classified in the family Enterobacteriaceae and is the only oxidase-positive member of this family (1). Freshwater and estuarine water are considered to be the natural environment of P. shigelloides, which is often isolated from fish and seafood (2, 3). P. shigelloides is a bacterium associated with diarrheal disease in humans (4). The organism has been reported to cause several types of gastroenteritis, including acute secretory gastroenteritis (5), an invasive shigellosis-like disease (6), and a cholera-like illness (7). Extraintestinal infections, such as meningitis, bacteremia (8), and pseudoappendicitis (9), are also associated with P. shigelloides infection. Unlike other phenotypic methods, serology has more successfully been used for distinguishing different strains of P. shigelloides. There are mainly two major serotyping schemes, which are based on somatic (O) and flagellar (H) antigens. Thus far, 102 somatic antigens and 51 flagellar antigens have been recognized (10).
Lipopolysaccharides (LPS), as in other members of the family Enterobacteriaceae, consist of three domains: an endotoxic glycolipid (lipid A), an O-polysaccharide (O-PS) chain or O antigen, and an intervening core oligosaccharide (core-OS) region. In studies of several Enterobacteriaceae-like Escherichia coli, Salmonella enterica, or Klebsiella pneumoniae strains, genes involved in LPS core biosynthesis are usually found clustered in a region of the chromosome, the waa gene cluster (11, 12). On the other hand, a careful analysis of several fully sequenced genomes suggested that genes for the LPS core biosynthesis may not be clustered and may be distributed between several regions, e.g., as in Yersinia pestis (13) or Proteus mirabilis (14).
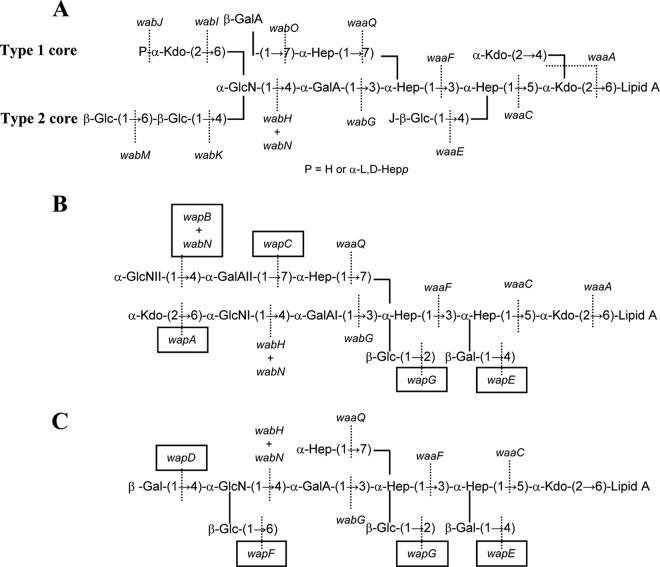
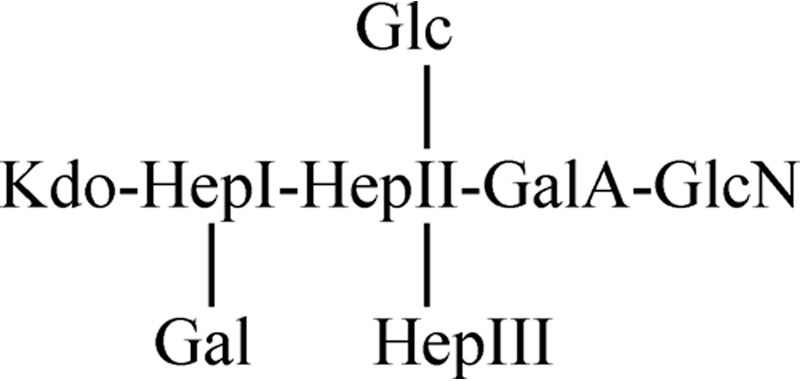
Despite the emerging importance of this pathogenic microorganism, only seven LPS structures of 102 O serotypes of P. shigelloides have been investigated to date. Thus far, only four complete LPS molecules isolated from P. shigelloides CNCTC 113/92 (serotype O54), CNCTC 144/92 (serotype O74) (15, 16), strain 302-73 (serotype O1) (17, 18), and recently CNCTC 80/89 (serotype O13) (19) have been elucidated. Figure 1 shows P. shigelloides LPS cores of strain 302-73 (serotype O1) and CNCTC 113/92 (serotype O54). P. shigelloides strain 7-63 (serotype O17) LPS shows the core oligosaccharide substituted with one repeating unit of the O-specific PS (20). It was known that its O-antigen structure is identical to that of Shigella sonnei phase I (21), a causative agent of dysentery. Both species acquired virulence plasmid with a gene cluster coding O17 antigen (22).
FIG 1.
LPS core chemical structures of K. pneumoniae type 1 (12) and 2 (27) (A) and P. shigelloides strains 302-73 (18) (B) and CNCTC 113/92 (15) (C).
The overwhelming majority of the LPS studied (23) contain at least one residue of 3-deoxy-d-manno-oct-2-ulosonic (ketodeoxyoctonic) acid (Kdo), which links the core to the lipid A moiety (KdoI). The second characteristic sugar of the core is l,d-Hep, although there are a few LPS that contain d,d-Hep or lack any heptose (24). In those containing l,d-Hep, the presence of a Hep-α-(1→5)-Kdo disaccharide is a characteristic feature. Two important features that completely differentiate enteric bacteria among them are the outer core disaccharide GalA-GlcN and the substitution of βGlc-HepI (Gal, galactose; Glc, glucose; GlcN, glucosamine), which are present in the Klebsiella-Serratia-Proteus group LPS and always absent in Escherichia-Salmonella-Shigella group LPS (12, 14, 25–27). The P. shigelloides LPS seems clearly to be included in the Klebsiella group according to this point (Fig. 1). P. shigelloides LPS shows a special feature because instead of the typical βGlc-HepI substitution found in the Klebsiella-Serratia-Proteus group LPS, is a βGal-HepI substitution (Fig. 1). We studied here for the first time the genetics of P. shigelloides LPS core in order to proceed with the complete gene assignment of all LPS core biosynthesis gene functions.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The bacterial strains and plasmids used in the present study are listed in Table 1. All strains were routinely grown in Luria-Bertani (LB) broth and LB agar (28) at 37°C unless stated otherwise. Ampicillin (100 and 150 μg ml−1, for E. coli and K. pneumoniae strains, respectively), chloramphenicol (25 μg ml−1), and kanamycin (25 μg ml−1) were added to the different media when required.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristics | Source or reference |
|---|---|---|
| Strains | ||
| P. shigelloides | ||
| 302-73 | Wild-type, serogroup 01 | 17 |
| CNCTC 113/92 | Wild-type, serogroup 054 | 15 |
| K. pneumoniae | ||
| 52145ΔwaaC | Nonpolar waaF mutant | 38 |
| 52145ΔwaaF | Nonpolar waaC mutant | 24 |
| 52145ΔwaaQ (NC19) | Nonpolar waaQ mutant | 27 |
| 52145ΔwaaE (NC16) | Nonpolar waaE mutant | 27 |
| 552145ΔwabO | Nonpolar wabO mutant | 40 |
| 52145ΔwabOΔwaaL | Nonpolar wabO and waaL double mutant | 40 |
| 52145ΔwabG | Nonpolar wabG mutant | 38 |
| 52145ΔwabH | Nonpolar wabH mutant | 39 |
| 52145ΔwabN | Nonpolar wabN mutant | 39 |
| 52145ΔwabK | Nonpolar wabK mutant | 27 |
| E. coli | ||
| DH5α | F− endA hsdR17 (rk− mk−) supE44 thi-1 recA1 gyrA96 ϕ80lacZ | 44 |
| CJB26 | waaA::kan recA harboring plasmid pJSC2 | 45 |
| S17-1 | hsdR pro recA, RP4-2 in chromosome Km::Tn7 (Tc::Mu) | 46 |
| Plasmids | ||
| pJSC2 | Cmr; temperature sensitive for replication containing E. coli waaA | 45 |
| pGEMT easy | PCR-generated DNA fragment cloning vector; Apr | Promega |
| pGEMT-WaaA302 | pGEM-T with waaA from strain 302-73; Apr | This study |
| pGEMT-WaaC302 | pGEM-T with waaC from strain 302-73; Apr | This study |
| pGEMT-WaaF302 | pGEM-T with waaF from strain 302-73; Apr | This study |
| pGEMT-WaaQ302 | pGEM-T with waaQ from strain 302-73; Apr | This study |
| pGEMT-WapE302 | pGEM-T with wapE from strain 302-73; Apr | This study |
| pGEMT-WabG302 | pGEM-T with wabG from strain 302-73; Apr | This study |
| pGEMT-WabH302 | pGEM-T with wabH from strain 302-73; Apr | This study |
| pGEMT-WabN302 | pGEM-T with wabN from strain 302-73; Apr | This study |
| pGEMT-WapA | pGEM-T with wapA from strain 302-73; Apr | This study |
| pGEMT-WapB | pGEM-T with wapB from strain 302-73; Apr | This study |
| pGEMT-WapC | pGEM-T with wapC from strain 302-73; Apr | This study |
| pGEMT-WapD | pGEM-T with wapD from strain CNCTC 113/92; Apr | This study |
| pGEMT-WapF | pGEM-T with wapF from strain CNCTC 113/92; Apr | This study |
| pGEMT-WapG | pGEM-T with wapG from strain302-73; Apr | This study |
| pGEMT-peg.367 | pGEMT with the peg.367 gene from strain 302-73; Apr | This study |
| pBAD33-Cm | Arabinose-inducible expression vector; Cmr | 46 |
| pBAD-WapB | Arabinose-inducible wapB from strain 302-73; Cmr | This study |
| pBAD-WapF | Arabinose-inducible wapF from strain CNCTC 113/92; Cmr | This study |
Cmr, chloramphenicol resistance; Apr, ampicillin resistance.
General DNA methods.
General DNA manipulations were accomplished essentially as previously described (28). DNA restriction endonucleases, T4 DNA ligase, E. coli DNA polymerase (Klenow fragment), and alkaline phosphatase were used as recommended by the suppliers.
DNA sequencing and computer analysis of sequence data.
Double-stranded DNA sequencing was performed by using the dideoxy-chain termination method (29) with the ABI Prism dye terminator cycle sequencing kit (Applied Biosystems). Oligonucleotides used for genomic DNA amplifications and DNA sequencing were purchased from Sigma-Aldrich. The DNA sequence was translated in all six frames, and all open reading frames (ORFs) were inspected. Deduced amino acid sequences were compared to those of DNA translated in all six frames from nonredundant GenBank and EMBL databases by using the BLAST (30) network service at the National Center for Biotechnology Information and the European Biotechnology Information. CLUSTAL W was used for multiple-sequence alignments (31).
Plasmid constructions and mutant complementation studies.
For complementation studies, the P. shigelloides 302-73 genes (waaA, waaC, waaF, wapE, wabG, wabH, wabN, wapA, wapB, wapC, wapG, and peg.367) and strain CNCTC 113/92 wapD and wapF were PCR amplified by using specific primer pairs (see the list of primers in Table 2) and chromosomal DNA as the template, ligated to plasmid pGEMT (Promega), and transformed into E. coli DH5α. Transformants were selected on LB plates containing ampicillin. Once checked plasmids with the amplified genes were independently transformed into K. pneumoniae core LPS mutants.
TABLE 2.
Primers used in this study to amplify and subclone individual genes
| Target gene | Primer | Sequence (5′–3′)a | PCR product size (bp) | Restriction tail |
|---|---|---|---|---|
| waaF | waaFPL-For | ATTCAAAACCGTGACCGAAG | 1,229 | |
| waaFPL-Rev | CATGGAGGAGGTTTTGACCA | 1,229 | ||
| waaC | waaCPL-For | CCGAGTAGCCCAGACTTCAC | 1,199 | |
| waaCPL-Rev | TGCCGATATGCTCTTGAATG | 1,199 | ||
| wapA | wapAPL-For | CCCGAATATCACGCTGTATG | 1,499 | |
| wapAPL-Rev | AAACCTATGGCGCAATAACC | 1,499 | ||
| wapC | wapCPL-For | TGACCCACTCCCCGTAGTAT | 1,401 | |
| wapCPL-Rev | CGGCATGATCCATTAAGACC | 1,401 | ||
| wapB | wapBPL-For | GCAGCATGCTCAGCAATTTA | 1,508 | |
| wapBPL-Rev | ATGCTGCACTCTTCCGAGTT | 1,508 | ||
| wapB | wapBPL-PBFor | TCTAGAGCAGCATGCTCAGCAATTTA | 1,508 | XbaI |
| wapBPL-PBRev | AAGCTTATGCTGCACTCTTCCGAGTT | 1,508 | SphI | |
| wabN | wabNPl-For | GGCATGCAAAATCATCACTG | 1,307 | |
| wabNPl-Rev | ACAAATCGCACAAGCGGTAT | 1,307 | ||
| waaQ | waaQPL-For | CGTACCGCCGTATAACTGGT | 1,367 | |
| waaQPL-Rev | ATTGACGGCATCATTCCATT | 1,367 | ||
| wabG | wabGPl-For | CCACGCCTTAGGGATTGATA | 1,900 | |
| wabGPl-Rev | ATCCGCTTGTAGCTGGGTAA | 1,900 | ||
| wabB | wabBPL-For | TATGCAGCGCGATGTCTTAG | 1,601 | |
| wabBPL-Rev | CGTGGGAATTACAGCAAACC | 1,601 | ||
| wapE | wapEPL-For | CACAGACGCAATCCAATCAG | 1,203 | |
| wapEPL-Rev | GATCTTAGCCGGTTCACTCG | 1,203 | ||
| waaA | waaAPL-For | CACCACGATGTCATCACACA | 1,620 | |
| waaAPL-Rev | ATTGGTGACCGGATCAAAAG | 1,620 | ||
| wapD | wapDPL-For | GCACAAGTTGCCTCCGTACT | 1,217 | |
| wapDPL-Rev | TCTTGTGGGGGTTGATTCAT | 1,217 | ||
| wapF | wapFPL-For | ACTCATTTGCCACCGATTTC | 1,209 | |
| wapFPL-Rev | TTCCGAGTTTATGCCTGGAG | 1,209 | ||
| wapF | wapFPL-PBFor | GTCGACACTCATTTGCCACCGATTTC | 1,209 | SalI |
| wapFPL-PBRev | AAGCTTTTCCGAGTTTATGCCTGGAG | 1,209 | HindIII | |
| wapG | wapGPL-For | ATGTACCGAGCCAACTACCG | 1,432 | |
| wapGPL-Rev | GCTTGTGCAGTGACAGGATG | 1,432 | ||
| peg.367 | gly367PL-For | TGCGGTTGGTGAGTTATCAG | 1,269 | |
| gly367PL-Rev | CTCGTTTCGTTCCGATTCTC | 1,269 |
Restriction sites are indicated by underlining.
Recombinant plasmids pBAD-WapB and pBAD-WapF were obtained by independent PCR amplification of these genes from P. shigelloides 302-73 and CNCTC 113/92, respectively, using primers with tails, subcloning in pBAD33-Cm, and transformation into E. coli DH5α. These constructs were transformed into K. pneumoniae core LPS mutants. wapB and wapF were expressed from the arabinose-inducible and glucose-repressible pBAD33-Cm promoter. Repression from the araC promoter was achieved by growth in medium containing 0.2% (wt/vol) d-glucose, and induction was obtained by adding l-arabinose to a final concentration of 0.2% (wt/vol). The cultures were grown for 18 h at 37°C in LB medium supplemented with chloramphenicol and 0.2% glucose, diluted 1:100 in fresh medium (without glucose), and grown until they reached an A600 of ∼0.2. Then, l-arabinose was added, and the cultures were grown for another 8 h. Repressed controls were maintained in glucose-containing medium.
LPS isolation and SDS-PAGE.
For screening purposes, LPS was obtained after proteinase K digestion of whole cells (32). LPS samples were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) or SDS-Tricine-PAGE and visualized by silver staining as previously described (32, 33).
Large-scale isolation and acid degradation of LPS.
Dry bacterial cells of each strain in 25 mM Tris-HCl buffer containing 2 mM CaCl2 (pH 7.63, 10 ml g−1) were treated at 37°C with RNase and DNase (24 h, 1 mg g−1 each) and then with proteinase K (36 h, 1 mg g−1). The suspension was dialyzed and lyophilized, and the LPS was extracted by either the phenol-water procedure (34) or the Galanos method (35). A portion of the LPS (50 mg) from each strain was heated with aqueous 2% acetic acid (6 ml) at 100°C for 45 min. The precipitate was removed by centrifugation (13,000 × g, 20 min), and the supernatant was fractionated on a column (56 by 2.6 cm) of Sephadex G-50 in 0.05 M pyridinium acetate buffer (pH 4.5) with monitoring by using a differential refractometer (Knauer, Berlin, Germany). Eluted fractions containing polysaccharide (positive in phenol-sulfuric acid assay) were extensively water dialyzed and lyophilized to concentrate core OS in strains devoid of the O-antigen LPS.
LPS chemical analysis.
For chemical analysis, either purified LPS or core LPS oligosaccharides samples were hydrolyzed with 1 N trifluoroacetic acid for 4 h at 100°C.
Alditol acetates and methyl glycoside acetates were analyzed on a Agilent Technologies 5973N MS instrument equipped with a 6850A gas chromatography (GC) and an RTX-5 capillary column (Restek; 30 m by 0.25 m [inner diameter]; flow rate, 1 ml min−1; He as the carrier gas).
Mass spectrometry studies.
Positive-ion reflection time-of-flight mass spectra (MALDI-TOF) were acquired on a Voyager DE-PR instrument (Applied Biosystems) equipped with a delayed extraction ion source. The ion acceleration voltage was 20 kV, the grid voltage was 14 kV, the mirror voltage ratio was 1.12, and the delay time was 100 ns. Samples were irradiated at a frequency of 5 Hz by 337-nm photons from a pulsed nitrogen laser. Mass calibration was obtained with a malto-oligosaccharide mixture from corn syrup (Sigma). A solution of 2,5-dihydroxybenzoic acid in 20% CH3CN in water at a concentration of 25 mg/ml was used as the MALDI matrix. A 1-μl portion of matrix solution and 1 μl of the sample were premixed and then deposited on the target. The droplet was allowed to dry at room temperature. Spectra were calibrated and processed under computer control using the Applied Biosystems Data Explorer software.
Dot blot hybridization.
The DNA probes used consisted of the digoxigenin-labeled amplification products of strains 302-73 and CNCTC 113/92 obtained with a DIG DNA labeling and detection kit (Boehringer, Mannheim, Germany). These 16 probes, one for each of the genes putatively involved in core LPS biosynthesis from both strains, were synthesized using the primer pairs shown in Table 2 for waaA, -C, -F, -Q, and -L, for wabN, -H, -G, and -O, and for wapA, -B, -C, -D, -E, -F, and -G. Cell lysates were obtained by resuspending the cells of 500-μl overnight cultures in LB medium in 100 μl of 0.4 M NaOH and heating the samples for 30 min at 80°C. One microliter of each cell lysate was blotted onto Hybond membranes and bound by UV cross-linking. Hybridization was performed according to a standard protocol (28) with stringent washing at 65°C in 0.2× SSC (20× SSC is 3 M NaCl plus 0.3 M sodium citrate; pH 7.0). Probes that remained bound to homologous sequences were detected with the DIG DNA labeling and detection kit according to the supplier's instructions.
Nucleotide sequence accession numbers.
The complete nucleotide sequences of the P. shigelloides 302-73 and CNCTC 113/92 gene clusters described here have been assigned to GenBank accession numbers KF648555 and KF648556, respectively.
RESULTS
Organization of the P. shigelloides 302-73 waa gene cluster.
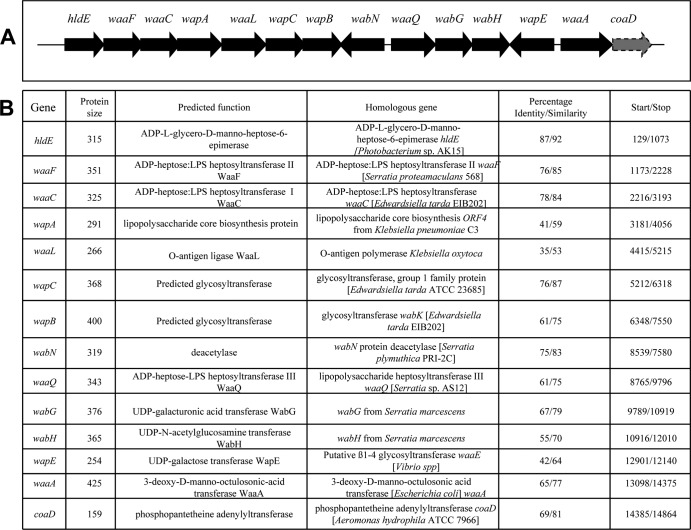
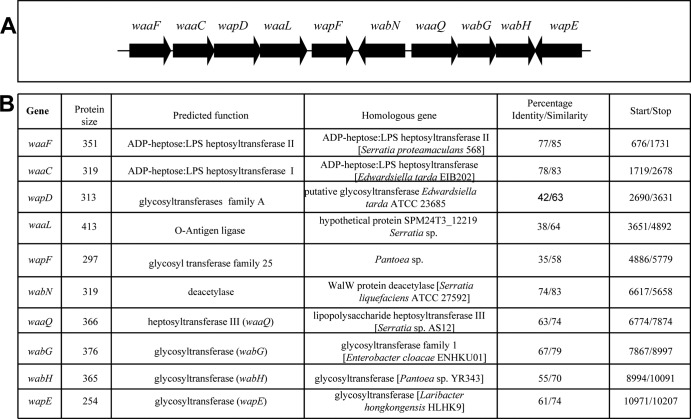
In most Enterobacteriaceae studied thus far, the genes involved in core LPS biosynthesis are found clustered (waa gene cluster) (12, 14, 23, 25, 27). Usually, the first gene of the cluster is hldE (ADP-d-glycero-d-manno-heptose epimerase) and at the 3′ end of the cluster the core biosynthesis unrelated genes coaD (phosphopantetheine adenylyltransferase) (36) are found. Recently, we sequenced the complete P. shigelloides 302-73 (serotype O1) genome (37), and hldE corresponds to peg.3153, and coaD corresponds to peg.3166. The complete waa cluster has been also separately sent to GenBank and is shown in Fig. 2.
FIG 2.
(A) Genetic organization of the chromosomal region (waa gene cluster) containing core LPS biosynthesis genes from P. shigelloides strain 302-73. (B) Characteristics of the genes and proteins of the ORFs in the P. shigelloides strain 302-73 waa.
P. shigelloides common inner and outer core genes.
The pentasaccharide l-α-HeppIII-(1→7)-l-α-HeppII-(1→3)-l-α-HeppI-(1→5)-[α-KdopII-(2→4)]-α-KdopI (23) has been found in all of the inner core regions of the studied Enterobacteriaceae. This pentasaccharide is biosynthesized by the sequential transfer to lipid A of one to two residues of Kdo by the CMP-Kdo:lipid A Kdo bifunctional transferase (WaaA), and three residues of l-heptose by ADP-heptose-heptosyltransferases I, II, and III (WaaC, WaaF, and WaaQ). The P. shigelloides homologue WaaA showed high levels of amino acid identity and similarity to the known WaaA from different Enterobacteriaceae (65 and 77%) (Fig. 2B). The WaaC, WaaF, and WaaQ homologues showed high levels of identity and similarity to the K. pneumoniae 52145 homologues WaaC (78 and 84%), WaaF (76 and 85%), and WaaQ (61 and 75%) (Fig. 2B).
Proper identification of the functions of these five inner core genes was performed as previously described by complementation studies of known inner-core mutants (25). A plasmid containing the waaA gene from P. shigelloides strain 302-73(pGEMT-WaaA302) was introduced into E. coli CJB26, a strain with a kanamycin resistance gene inserted in the chromosomal waaA and harboring a wild-type waaA in a temperature-sensitive plasmid (pJSC2). The pGEMT-WaaA302 plasmid restored the growth at 44°C of the CJB26 mutant. Analysis of LPS by SDS-Tricine-PAGE showed that K. pneumoniae 52145 mutant strains 52145ΔwaaC, 52145ΔwaaF, and 52145ΔwaaQ (27) were complemented by plasmids pGEMT-WaaC302, pGEMT-WaaF302, and pGEMT-WaaQ302, respectively (Fig. 3).
FIG 3.
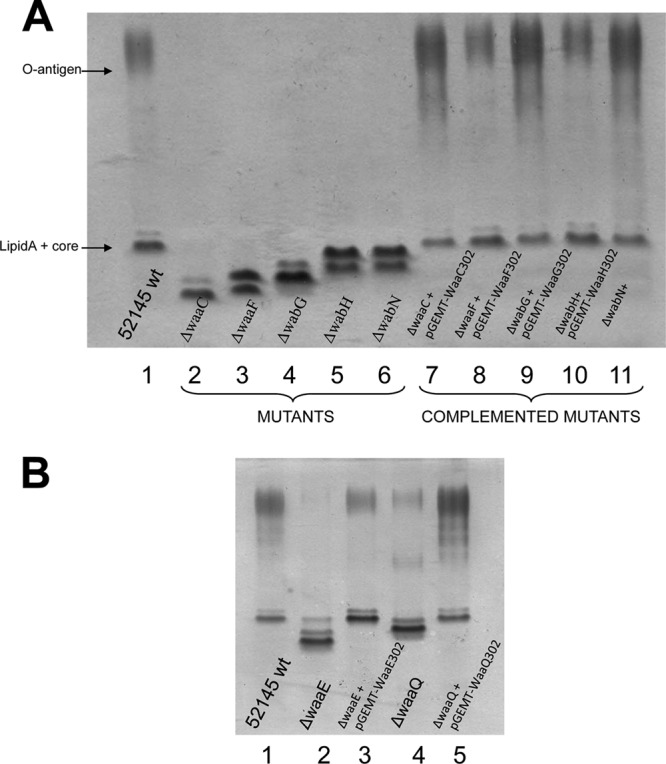
Tricine-SDS-PAGE analysis of K. pneumoniae LPS samples. (A) Lanes: 1, 52145 (wild type); 2, 52145ΔwaaC; 3, 52145ΔwaaF; 4, 52145ΔwabG; 5, 52145ΔwabH; 6, 52145ΔwabN; 7, 52145ΔwaaC(pGEMT-WaaC302); 8, 52145ΔwaaF(pGEMT-WaaF302); 9, 52145ΔwabG(pGEMT-WabG302); 10, 52145ΔwabH(pGEMT-WabH302); and 11, 52145ΔwabN(pGEMT-WabN302). (B) Lanes: 1, 52145 (wild type); 2, 52145ΔwaaE; 3, 52145ΔwaaE(pGEMT-WaaE302); 4, 52145ΔwaaQ; and 5, 52145ΔwaaQ(pGEMT-WaaQ302).
The outer core disaccharide αGlcNI-(1→4)-GalAI is another common feature shared by K. pneumoniae (12, 14, 25, 26, 27) and P. shigelloides 302-73 (18) (Fig. 1). We have previously shown that the K. pneumoniae WabG is responsible for the transfer of GalA to the O-3 position of l-glycero-d-manno-heptose II (L-HepII) (38). Similarly, we have shown that two K. pneumoniae enzymes (WabH and WabN) are required for the incorporation of the GlcN residue. The WabH transfers a d-N-acetylglucosamine (d-GlcNAc) residue from UDP-GlcNAc to the first outer-core residue GalA and WabN deacetylates the GlcNAc residue to GlcN (39). K. pneumoniae mutants 52145ΔwabG, 52145ΔwabH, and 52145ΔwabN produce shorter core LPS than wild-type strain 52145, and their LPS is devoid of O-antigen LPS. As expected, pGEMT-WabG302, pGEMT-WabH302, and pGEMT-WabN302 were able to restore wild-type O-antigen LPS production when introduced into 52145ΔwabG, 52145ΔwabH, and 52145ΔwabN, respectively (Fig. 3A). Compositional analysis of the core OS fractions released by acid hydrolysis and GC studies as described in Materials and Methods showed the presence of GlcNAc and GlcN in strains 52145ΔwabH(pGEMT-WabH302) and 52145ΔwabN(pGEMT-WabN302), respectively. In contrast, OS fraction from strain 52145ΔwabH lacks either GlcNAc or GlcN; and strain 52145ΔwabN shows GlcNAc instead of GlcN, as previously reported (38, 39). Furthermore, a similar analysis of the OS fractions from strain 52145ΔwabG(pGEMT-WabG302) showed the presence of GalA, whereas this residue was absent from strain 52145ΔwabG. These results show that these three genes are functional homologues of the K. pneumoniae ones.
Common but functionally different P. shigelloides 302-73 inner-core gene.
The ORF12 shows a clear domain β1-4 glycosyltransferase being from the GT2 family and named wapE (Fig. 2). It shows homology to WaaE (26) from different Vibrio species (42 and 64% identity and similarity, respectively) and also to K. pneumoniae WaaE (30 and 48% identity and similarity, respectively). The βGlc-(1→4) linked to the l-HepI, as mentioned in the introduction, is an inner-core characteristic of different enteric species from the Klebsiella-Serratia group, but this motif is not present in P. shigelloides 302-73 LPS core. Instead, in P. shigelloides 302-73 LPS core there is a βGal-(1→4) linked to the l-HepI (Fig. 1). To determine the function of this gene, we introduced it into K. pneumoniae 52145ΔwaaE. The K. pneumoniae 52145ΔwaaE LPS profile in SDS-Tricine-PAGE gels showed a great reduction of the O-antigen LPS bands and a clear increase in the migration of the LPS core band. The introduction of pGEMT-WapE302 into mutant 52145ΔwaaE resulted in a LPS profile as the wild-type strain with multiple O-antigen bands and the recovery of the LPS core band migration (Fig. 3B). Chemical analysis of core oligosaccharides isolated from LPS of strain 52145ΔwaaE(pGEMT-WapE302) showed the presence of galactose, whereas no such monosaccharide could be found in the LPS core oligosaccharides isolated from strain 52145ΔwaaE.
Specific P. shigelloides 302-73 outer-core genes.
The remaining genes in the 302-73 waa gene cluster (orf4, -6, and -7, named wapA, wapB, and wapC, respectively) were expected to be involved in outer-core completion. BLAST search (Fig. 2B) of the encoded putative protein from wapA showed similarity (59%) and identity (41%) to the ORF4 of the K. pneumoniae C3 waa cluster that putatively links the terminal Kdo to the GlcNI in the outer-core LPS type 1 (12). No protein domains of glycosyl transferase could be found in this protein. The WapB encodes a glycosyl transferase from family 1 that showed similarity (46%) and identity (30%) to the well-characterized WabK (Fig. 2B). This protein in K. pneumoniae LPS core type 2 links the first Glc residue to the GlcN in the outer core; however, no βGlc-(1→4)-αGlcN motif is present in the LPS core of P. shigelloides 302-73.
BLAST search (Fig. 2B) of the encoded putative protein from wapC do not render any homology to well-characterized proteins with a domain highly characteristic from the glycosyl transferase family 1. This protein additionally shows a glycosyl transferase family 4. To determine the function of these three genes, we decided to introduce them into K. pneumoniae 52145ΔwabK (38) and 52145ΔwabOΔwaaL (40) mutants because these genes produce truncated core LPS extending up to the outer core GlcNI and l-HepIII residues, respectively, without O-antigen molecules. We expected that these mutant LPS could be good acceptors for residues transferred by some of the proteins encoded by these three P. shigelloides 302-73 genes.
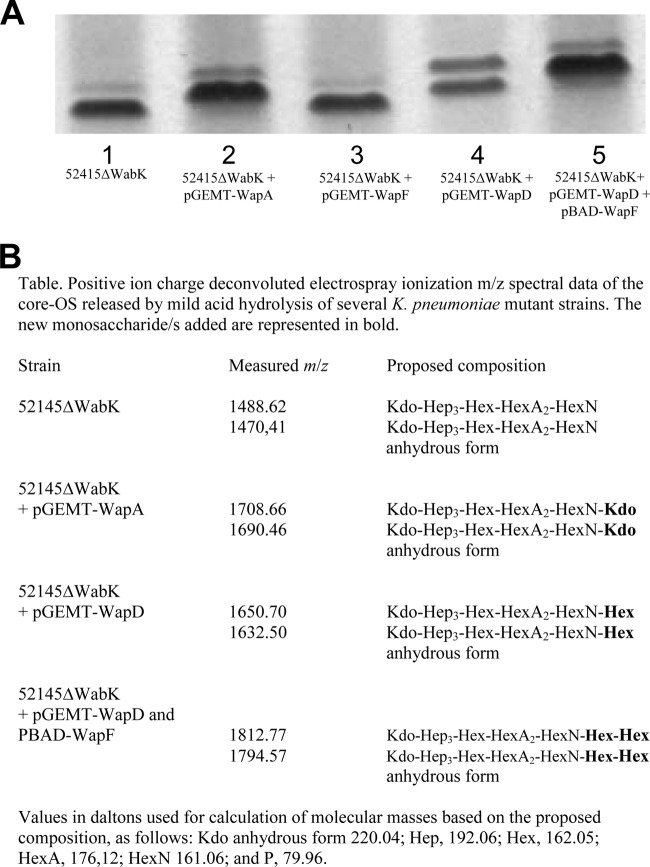
LPS from K. pneumoniae 52145ΔwabK analyzed by SDS-Tricine-PAGE showed an increase in mobility compared to that of wild-type 52145 LPS (Fig. 4A). Introduction of pGEMT-WapA302 into mutant 52145ΔwabK resulted in LPS with a decrease in mobility compared to that of 52145ΔwabK (Fig. 4A). No differences could be observed between 52145ΔwabK and 52145ΔwabK with plasmid vector pGEMT alone. LPS was extracted and purified from strains 52145ΔwabK and 52145ΔwabK(pGEMT-WapA302), the corresponding core oligosaccharide (OS) fractions were obtained by acid hydrolysis (see Materials and Methods), and MALDI-TOF spectra in the positive mode were obtained. Major signals at m/z 1,488.62 and 1,470.41 were obtained from strain 52145ΔwabK corresponding to Kdo-Hep3-Hex-HexA2-HexN and its anhydrous form, respectively (27) (Fig. 4B). The OS fraction of LPS from 52145ΔwabK(pGEMT-WapA) showed major signals at m/z 1,708.66 and 1,690.46 that were ∼220.05 Da (anhydrous Kdo) higher than those obtained from 52145ΔwabK (Fig. 4B). No changes were observed when pGEMT-WapA was introduced in other mutants, such as 52145ΔwabH (27), analyzed by SDS-Tricine-PAGE. These data suggest that WapA is responsible for the addition of Kdo to the GlcNI in the outer-core LPS of P. shigelloides 302-73 (Fig. 1).
FIG 4.
(A) Tricine-SDS-PAGE analysis of LPS samples from K. pneumoniae strains 52145ΔwabK (lane 1), 52145ΔwabK(pGEMT-WapA) (lane 2), 52145ΔwabK(pGEMT-WapF) (lane 3), 52145ΔwabK(pGEMT-WapD) (lane 4), and 52145ΔwabK(pGEMT-WapD, pBAD-WapF) (lane 5) grown under inducing conditions (arabinose, 0.2%). (B) m/z spectrum data for the core OS from strains that modify the K. pneumoniae 52145ΔwabK mutant.
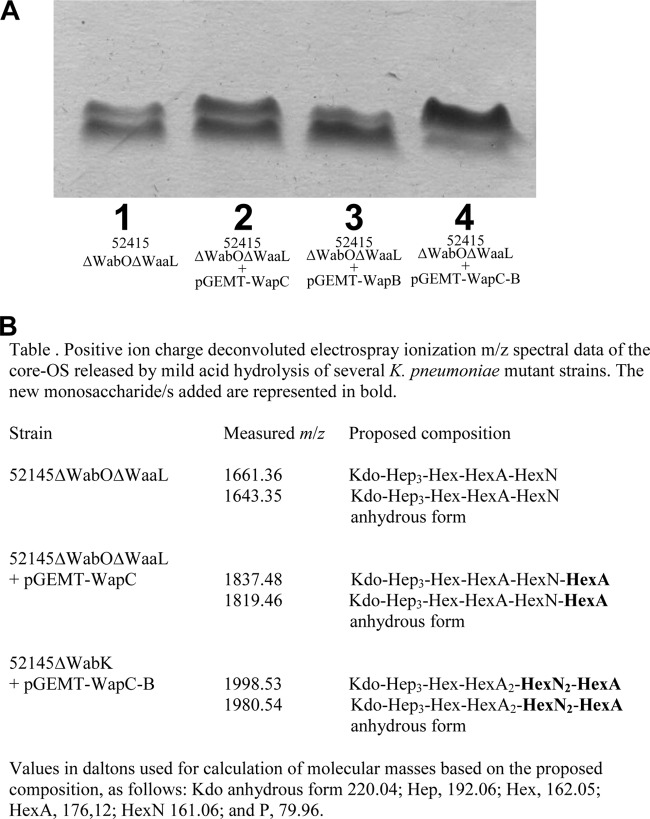
Because we already found the genes responsible for the production of the main branch of OS core Kdo-HepI-HepII-GalAI-GlcNI-Kdo, we decided to study using the 52145ΔwabOΔwaaL mutant the possible branched motif GlcNII-α-(1→4)-GalAII-α-(1→7)-l-HepIII (see Fig. 1C). When pGEMT-WapC was introduced into 52145ΔwabOΔwaaL mutant (40), migration of LPS in SDS-Tricine-PAGE was modified (Fig. 5A) but not with pGEMT-WapB, suggesting that core LPS extending up to the l-HepIII residue could not act as acceptor of WapB transferase. MALDI-TOF analysis of the OS from the 52145ΔwabOΔwaaL (lacking the O-antigen molecules) mutant showed two main peaks at m/z 1,661.36 and 1,643.35, which correspond to the pseudomolecular ions (M+Na)+ and (M+Na-18)+, respectively (40) (Fig. 5B). The signal at m/z 1,661.36 was in agreement with the calculated average molecular mass 1,638.42 Da of an OS structure with one hexose, three heptoses, one hexuronic acid, one hexosamine, and one Kdo unit. MALDI-TOF analysis of the OS from 52145ΔwabOΔwaaL(pGEMT-WapC) showed major signals at m/z 1,837.48 and 1,819.46 (Fig. 5B). These signals were in agreement with Kdo-Hep3-Hex-HexN-HexA2 and its anhydrous form and with the incorporation of one HexA.
FIG 5.
(A) Tricine-SDS-PAGE analysis of LPS samples from K. pneumoniae strains 52145ΔwabOΔwaaL (lane 1), 52145ΔwabOΔwaaL(pGEMT-WapC) (lane 2), 52145ΔwabOΔwaaL(pGEMT-WapB) (lane 3), and 52145ΔwabOΔwaaL(pGEMT-WapC, pBAD-WapB) (lane 4) grown under inducing conditions (arabinose, 0.2%). (B) m/z spectrum data for the core OS from strains that modify the K. pneumoniae 52145ΔwabOΔwaaL mutant.
When we introduced WapB in 52145ΔwabOΔwaaL, together with WapC (pGEMT-WapC and pBAD-WapB), under inducing conditions a decrease in the migration of LPS in SDS-Tricine-PAGE (Fig. 5A) could be observed. This decrease was higher than the WapC alone modification. MALDI-TOF analysis of the OS from 52145ΔwabOΔwaaL(pGEMT-WapC, pBAD-WapB) grown under inducing conditions showed major signals at m/z 1,998.53 and 1,980.54 (Fig. 5B). These signals were in agreement with Kdo-Hep3-Hex-HexN2 -HexA2 and its anhydrous form and with the incorporation of one HexA and one HexN.
These results strongly suggest that WapC is responsible for the linkage of GalAII to HepIII (UDP-galacturonic acid transferase). WapB is responsible for the linkage of GlcNII to GalAII (UDP-N-acetyl-glucosaminyltransferase), whereas WabN deacetylates the core OS containing GlcNAc residue to GlcN (see Fig. 1).
Organization and characterization of the P. shigelloides CNCTC 113/92 waa gene cluster.
Using primers defined according to the P. shigelloides 302-73 (serotype O1) waa cluster, we were able to complete the DNA sequence of the P. shigelloides strain CNCTC 113/92 (serotype O54) waa cluster (Fig. 6A). When we analyzed this waa cluster, we observed two different glycosyltransferases (named wapD and wapF in the Fig. 6A) from the P. shigelloides 302-73 waa cluster (Fig. 2). The rest of the waa genes were >95% homologues with the respective ones from P. shigelloides 302-73 waa, and hldE and waaA were flanking the genes shown in the Fig. 6A (data not shown). pGEMT-WapD introduced into 52145ΔwabK mutant modify the migration of LPS in SDS-Tricine-PAGE (Fig. 4A) but not pGEMT-WapF, suggesting that core LPS extending up to the GlcNI residue could not act as an acceptor of WapF transferase. MALDI-TOF analysis of the OS from 52145ΔwabK mutant has been previously mentioned and characterized (27).The OS fraction of LPS from 52145ΔwabK(pGEMT-WapD) showed major signals at m/z 1,650.70 and 1,632.50, ∼162.08 Da (one hexose residue) higher than those obtained from 52145ΔwabK (Fig. 4B). The chemical analysis of core oligosaccharides isolated from LPS of strain 52145ΔwaaK(pGEMT-WapD) showed the presence of galactose, whereas no such monosaccharide could be found in the 52145ΔwabK LPS core.
FIG 6.
(A) Genetic organization of the chromosomal region (waa gene cluster) containing core LPS biosynthesis genes from P. shigelloides strain CNCTC 113/92 (serotype O54). (B) Characteristics of the genes and proteins of the distinctive ORFs in the P. shigelloides strain CNCTC 113/92 waa.
The introduction of WapF in 52145ΔwabK, together with WapD (pGEMT-WapD and pBAD-WapF), under inducing conditions provoked a decrease in the migration of LPS in SDS-Tricine-PAGE (Fig. 4A). Such decrease could be observed either versus LPS of 52145ΔwabK or 52145ΔwabK(pGEMT-WapD). MALDI-TOF analysis of the OS from 52145ΔwabK(pGEMT-WapD, pBAD-WapF) grown under inducing conditions showed major signals at m/z 1,812.77 and 1,794.57 (Fig. 4B). These signals were in agreement with the chemical structure of Kdo-Hep3-Hex3-HexN-HexA and its anhydrous form and with the incorporation of two hexose residues. These results seems to indicate that WapD is the β-(1→4) galactosyltransferase to the GlcN and that WapF is the β-(1→6) glucosyltransferase to the GlcN, which is in agreement with the LPS core chemical structure of P. shigelloides CNCTC 113/92 strain (serotype O54) (Fig. 1C).
Specific P. shigelloides gene outside the waa cluster.
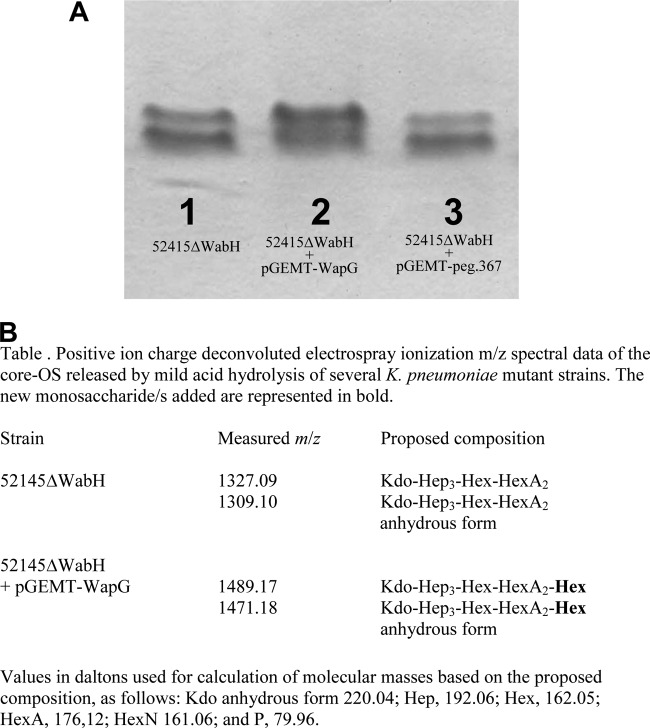
The P. shigelloides LPS motif βGlc-(1→2)-α-l-HepII seems not be encoded by any of the glycosyltransferases found in both waa clusters. For this reason, we decided to analyze all of the P. shigelloides 302-73 (serotype O1) genome (37) for a possible candidate gene. We found two putative candidates (peg.1453 and peg.367), but only the first of them (named wapG) was able to modify the gel migration of LPS from the 52145ΔwabH mutant (27, 39) when introduced using pGEMT-WapG (Fig. 7A).
FIG 7.
(A) Tricine-SDS-PAGE analysis of LPS samples from K. pneumoniae strains 52145ΔwabH (lane 1), 52145ΔwabH(pGEMT-WapG) (lane 2), and 52145ΔwabH(pGEMT-peg.367) (lane 3). (B) m/z spectrum data for the core OS from strains that modify the K. pneumoniae 52145ΔwabH mutant.
The OS from strains 52145ΔwabH and 52145ΔwabH(pGEMT-WapG) were obtained, and MALDI-TOF spectra in the positive mode performed. Major signals at m/z 1,327.09 and 1,309.10 were obtained from 52145ΔwabH corresponding to Kdo-Hep3-Hex-HexA2 and its anhydrous form, respectively (Fig. 7B). In agreement with the presence of a Hex residue, the OS fraction of LPS from 52145ΔwabH(pGEMT-WapG) showed major signals at m/z 1,489.17 and 1,471.18, ∼162.08 Da higher (one hexose residue) than those obtained from 52145ΔwabH (Fig. 7B). The pGEMT-WapG was unable to modify the gel migration of LPS from a mutant that lacks l-HepII (52145ΔwaaF) (12, 27).
Distribution of core biosynthetic genes in P. shigelloides.
To determine the degree of conservation of the genes putatively involved in P. shigelloides core LPS biosynthesis, specific digoxigenin-labeled PCR amplification probes were used in dot blot hybridization experiments. Sixteen probes, one for each of the genes putatively involved in core LPS biosynthesis from both strains, were synthesized using the primer pairs shown in Table 2 for waaA, -C, -F, -Q, and -L, for wabN, -H, -G, and -O, and for wapA, -B, -C, -D, -E, -F, and -G. The 16 probes were used in dot blot assays to screen genomic DNA from 12 P. shigelloides strains from our lab. Eight strains represented five different serotypes (O1, O2, O3, O17, and O54) plus four nonserotyped strains; seven were isolated from clinical stools and five from fish, with geographical origins from Japan (4), Brazil (3), Poland (1), and Spain (4). All of the P. shigelloides genomic DNA reacted with probes for waaA, -C, -F, and -Q, for wabN, -H, and -G, and for wapE and -G, suggesting that genes involved in the biosynthesis of the core LPS up to the outer residue GlcNI (see Fig. 1B) are conserved in this bacterial genus with a single species. The two O1 strains reacted with wapA and were the only strains to react. Four strains reacted with wapB and -C, the two O1 strains, the O13 strain, and one nonserotyped strain. wapD and -F reacted with two nonserotyped strains.
DISCUSSION
In this study we have been able to identify the functions of the genes found in the waa gene cluster from two P. shigelloides strains (302-73 and CNCTC 113/92, serotypes O1 and O54, respectively). The approach used for such identification was based in complementation studies for genes with homologues of known function. For the remaining genes, we took advantage of the fact that the P. shigelloides core LPS structure is highly similar to that of K. pneumoniae at least up to the outer-core residue GlcNI (Fig. 1). The functions of these genes were identified by using as surrogate acceptors LPS from well-defined K. pneumoniae core LPS mutants. This approach, unlike mutagenesis studies, has the advantage that we look for a positive trait (the addition to the mutant LPS of a particular residue) with an acceptor nearly identical to the natural LPS acceptor.
Both P. shigelloides waa clusters shared all of the genes besides the ones flanking waaL. In strain 302-73, orf4 corresponds to wapA and after the waaL, two other genes could be found (orf6 and -7, named wapB and -C, respectively). In strain CNCTC 113/92, orf4 corresponds to wapD, and orf6 (a single gene after waaL) corresponds to wapF (Fig. 2 and 6). We have been able to assign a proteomic function to all of these genes not shared by both strains. The data obtained seems to indicate that WapA is a CMP-Kdo transferase α-(2→6) to GlcNI, WapB is a UDP-N-acetylglucosamine transferase α-(1→4) to GalAII, WapC is a UDP-galacturonic acid transferase α-(1→7) to l-HepIII, in agreement with the LPS core chemical structure of P. shigelloides 302-73 (Fig. 1B). WapD is a UDP-galactose transferase β-(1→4) to GlcN, and WapF is a UDP-glucose transferase β-(1→6) to GlcN, in agreement with the LPS core chemical structure of P. shigelloides strain CNCTC 113/92 (Fig. 1C). It is important to note the complete lack of homology between the WapC and the K. pneumoniae WabO (40), which are both UDP-galacturonic acid transferases to l-HepIII, the first one α-(1→7) and the second one β-(1→7) (see Fig. 1A and B).
The E. coli core OS structures share a substructure up to the first outer-core residue (GlcI) (41). In E. coli strains with core OS types K-12, R1, R2, and R4, the WaaO glucosyltransferase links a second Glc residue (GlcII) to GlcI by an α-(1→2) linkage (41). In strains with an R3 core type, the WaaI galactosyltransferase links a Gal residue to GlcI with the same α-(1→2) linkage (41). Both WaaO and WaaI belong to the GT-A fold containing the CAZY glycosyltransferase family 8. An alignment between these two glycosyltransferases (WaaO, Q8KMW9; WaaI, Q9ZIT4) revealed that they share 51 and 80% amino acid residue identity and similarity, respectively. These levels of identity/similarity are similar to the ones shared by the P. mirabilis N-acetylglucosaminyl (WabHR110) and N-acetylgalactosaminyl (WabP50/57) transferases (42). P. shigelloides WapE is a UDP-galactose transferase β-(1→4) to l-HepI instead of the UDP-glucose transferase β-(1→4) to l-HepI characteristic of the Klebsiella-Serratia-Proteus group. In our case, K. pneumoniae WaaE and P. shigelloides WapE share 42 and 64% identity and similarity; it appears that glycosyltransferases involved in the transfer of different sugar epimer residues to the same LPS acceptor can be differentiated by their levels of identity and similarity but are still noticeable, unlike what happens with different linkages (α or β).
We previously showed that the incorporation of GlcN in the LPS core requires two distinct enzymatic steps due to the lack of UDP-GlcN in prokaryotes (39, 42). First, one enzyme catalyzes the incorporation of GlcNAc from UDP-GlcNAc to outer-core LPS, and then a second enzyme catalyzes the deacetylation of the core LPS containing the GlcNAc residue. Because we found an additional HexN (named GlcNII in Fig. 1B) when we analyzed the LPS of mutant 52145ΔwabOΔwaaL(pGEMT-WapC, pBAD-WapB) grown under inducing conditions but not a HexNAc, and we could not find any other deacetylase similar to WabN in the P. shigelloides 302-73 full genome (37), we suggested that the unique WabN present is able to deacetylate both GlcN residues (I and II in Fig. 1B). Located outside the waa cluster, wapG encodes the UDP-glucose transferase β-(1→2) to l-HepII, and it is an LPS core motif conserved in all of the P. shigelloides strains studied until now (15–19) (Fig. 1). The chromosome location seems to be the same in strains 302-73 and CNCTC 113/92 (data not shown). The waa gene cluster is located in a single chromosome location in several Enterobacteriaceae (11, 12). However, Proteus or Yersinia shows two or more chromosome locations for waa genes (13, 14). The case of Plesiomonas waa genes seems to be intermediate. All of the waa genes are in the same chromosome location besides a single waa gene that is located in another chromosome region.
The dot blot hybridization experiments seem to suggest that the P. shigelloides strains mainly share the following LPS core chemical structure:
 |
However, it is described that one strain from serotype O74 (16) lacks the βGlc-(1→2) linked to the l-HepII, the βGal-(1→4) linked to the l-HepI, or the αGlcN-(1→4) linked to the GalA but, unfortunately, we do not have any P. shigelloides strain belonging to this serotype.
Homologous recombination in housekeeping genes affects P. shigelloides alleles and nucleotides 7 and 77 times more frequently than mutation, respectively (43). These ratios are similar to those observed in the naturally transformable species Streptococcus pneumoniae with a high rate of recombination. In contrast, recombination within Enterobacteriaceae was much less frequent. The high recombination observed in this bacterium could explain why P. shigelloides showed an important variety of LPS core structures besides being single species on the genus. It could be observed with a higher variability on genes flanking waaL than in the rest of the waa cluster. These genes encoded the most external LPS core residues, where the variability is also wider than in the rest of the LPS core. A similar situation is responsible for the K. pneumoniae two type of LPS core, genes flanking the waaL in the waa cluster (27).
ACKNOWLEDGMENTS
This study was supported by Plan Nacional de I+D+i (Ministerio de Educación, Ciencia y Deporte and Ministerio de Sanidad, Spain) and by the Generalitat de Catalunya (Centre de Referència en Biotecnologia).
We thank Maite Polo for technical assistance and the Servicios Científico-Técnicos of the University of Barcelona.
Footnotes
Published ahead of print 15 November 2013
This article is dedicated to Miguel Regué.
REFERENCES
- 1.Janda JM. 2005. Family I. Enterobacteriaceae, genus XXVII. Plesiomonas Habs and Schubert 1962, p 740–744 In Brenner DJ, Krieg NR, Staley JT. (ed), Bergey's manual of systematic bacteriology, 2nd ed, vol 2 Springer, New York, NY [Google Scholar]
- 2.Herrera FC, Santos JA, Otero A, Garcia-Lopez ML. 2006. Occurrence of Plesiomonas shigelloides in displayed portions of saltwater fish determined by a PCR assay based on the hugA gene. Int. J. Food Microbiol. 108:233–238. 10.1016/j.ijfoodmicro.2005.12.008 [DOI] [PubMed] [Google Scholar]
- 3.Martinez-Murcia AJ, Benlloch S, Collins MD. 1992. Phylogenetic interrelationships of members of the genera Aeromonas and Plesiomonas as determined by 16S ribosomal DNA sequencing: lack of congruence with results of DNA-DNA hybridizations. Int. J. Syst. Bacteriol. 42:412–421. 10.1099/00207713-42-3-412 [DOI] [PubMed] [Google Scholar]
- 4.Brenden RA, Miller MA, Janda JM. 1988. Clinical disease spectrum and pathogenic factors associated with Plesiomonas shigelloides infections in humans. Rev. Infect. Dis. 10:303–316. 10.1093/clinids/10.2.303 [DOI] [PubMed] [Google Scholar]
- 5.Mandal BK, Whale K, Morrison BC. 1982. Acute colitis due to Plesiomonas shigelloides. BMJ 285:1539–1540. 10.1136/bmj.285.6354.1539-a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McNeeley D, Ivy P, Craft JC, Cohen I. 1984. Plesiomonas: biology of the organism and diseases in children. Pediatr. Infect. Dis. J. 3:176–181. 10.1097/00006454-198403000-00023 [DOI] [PubMed] [Google Scholar]
- 7.Tsukamoto T, Kinoshita Y, Shimada T, Sakazaki R. 1978. Two epidemics of diarrhoeal disease possibly caused by Plesiomonas shigelloides. J. Hyg. 80:275–280. 10.1017/S0022172400053638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Billiet J, Kuypers S, Van Lierde S, Verhaegen J. 1989. Plesiomonas shigelloides meningitis and septicemia in a neonate: report of a case and review of the literature. J. Infect. 19:267–271. 10.1016/S0163-4453(89)90809-8 [DOI] [PubMed] [Google Scholar]
- 9.Fischer K, Chakraborty T, Hof H, Kirchner T, Wamsler O. 1988. Pseudo-appendicitis caused by Plesiomonas shigelloides. J. Clin. Microbiol. 26:2675–2677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aldova E, Shimada T. 2000. New O and H antigens of the International Antigenic Scheme for Plesiomonas shigelloides. Folia Microbiol. 45:301–304. 10.1007/BF02817550 [DOI] [PubMed] [Google Scholar]
- 11.Heinrichs DE, Yethon JA, Whitfield C. 1998. Molecular basis for structural diversity in the core regions of the lipopolysaccharides of Escherichia coli and Salmonella enterica. Mol. Microbiol. 30:221–232. 10.1046/j.1365-2958.1998.01063.x [DOI] [PubMed] [Google Scholar]
- 12.Regué M, Climent N, Abitiu N, Coderch N, Merino S, Izquierdo L, Altarriba M, Tomás JM. 2001. Genetic characterization of the Klebsiella pneumoniae waa gene cluster, involved in core lipopolysaccharide biosynthesis. J. Bacteriol. 183:3564–3573. 10.1128/JB.183.12.3564-3573.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knirel YA, Dentovskaya SV, Bystrova OV, Kocharova NA, Senchenkova SN, Shaikhutdinova RZ, Titareva GM, Bakhteeva IV, Lindner B, Pier GB, Anisimov AP. 2007. Relationship of the lipopolysaccharide structure of Yersinia pestis to resistance to antimicrobial factors. Adv. Exp. Med. Biol. 603:88–96. 10.1007/978-0-387-72124-8_7 [DOI] [PubMed] [Google Scholar]
- 14.Aquilini E, Azevedo J, Jimenez N, Bouamama L, Tomás JM, Regué M. 2010. Functional identification of the Proteus mirabilis core lipopolysaccharide biosynthetic genes. J. Bacteriol. 192:4413–4424. 10.1128/JB.00494-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Niedziela T, Lukasiewicz J, Jachymek W, Dzieciatkowska M, Lugowski C, Kenne L. 2002. Core oligosaccharides of Plesiomonas shigelloides O54:H2 (strain CNCTC 113/92): structural and serological analysis of the lipopolysaccharide core region, the O-antigen biological repeating unit, and the linkage between them. J. Biol. Chem. 277:11653–11663. 10.1074/jbc.M111885200 [DOI] [PubMed] [Google Scholar]
- 16.Niedziela T, Dag S, Lukasiewicz J, Dzieciatkowska M, Jachymek W, Lugowski C, Kenne L. 2006. Complete lipopolysaccharide of Plesiomonas shigelloides O74:H5 (strain CNCTC 144/92). 1. Structural analysis of the highly hydrophobic lipopolysaccharide, including the O-antigen, its biological repeating unit, the core oligosaccharide, and the linkage between them. Biochemistry 45:10422–10433. 10.1021/bi0607709 [DOI] [PubMed] [Google Scholar]
- 17.Pieretti G, Corsaro MM, Lanzetta R, Parrilli M, Canals R, Merino S, Tomás JM. 2008. Structural studies of the O-chain polysaccharide from Plesiomonas shigelloides strain 302-73 (serotype O1). Eur. J. Org. Chem. 8:3149–3155. 10.1002/ejoc.200800198 [DOI] [Google Scholar]
- 18.Pieretti G, Carillo S, Lindner B, Lanzetta R, Parrilli M, Vilches S, Merino S, Tomás JM. 2010. The complete structure of the core of the LPS from Plesiomonas shigelloides 302-73 and the identification of its O-antigen biological repeating unit. Carbohydr. Res. 345:2523–2528. 10.1016/j.carres.2010.09.007 [DOI] [PubMed] [Google Scholar]
- 19.Kaszowska M, Jachymek W, Niedziela T, Koj S, Kenne L, Lugowski C. 2013. The novel structure of the core oligosaccharide backbone of the lipopolysaccharide from the Plesiomonas shigelloides strain CNCTC 80/89 (serotype O13). Carbohydr. Res. 380:45–50. 10.1016/j.carres.2013.07.005 [DOI] [PubMed] [Google Scholar]
- 20.Kubler-Kielb J, Mocca C, Vinogradov E. 2008. The elucidation of the structure of the core part of the LPS from Plesiomonas shigelloides serotype O17 expressing O-polysaccharide chain identical to the Shigella sonnei O-chain. Carbohydr. Res. 343:3123–3127. 10.1016/j.carres.2008.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Batta G, Liptak A, Schneerson R, Pozsgay V. 1997. Conformational stabilization of the altruronic acid residue in the O-specific polysaccharide of Shigella sonnei/Plesiomonas shigelloides. Carbohydr. Res. 305:93–99. 10.1016/S0008-6215(97)00197-3 [DOI] [PubMed] [Google Scholar]
- 22.Shepherd JG, Wang L, Reeves PR. 2000. Comparison of O-antigen gene clusters of Escherichia coli (Shigella) sonnei and Plesiomonas shigelloides O17: sonnei gained its current plasmid-borne O-antigen genes from P. shigelloides in a recent event. Infect. Immun. 68:6056–6061. 10.1128/IAI.68.10.6056-6061.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holst O. 2002. Chemical structure of the core region of lipopolysaccharides: an update. Trends Glycosci. Glycotechnol. 14:87–103. 10.4052/tigg.14.87 [DOI] [Google Scholar]
- 24.Caroff M, Karibian D. 2003. Structure of bacterial lipopolysaccharides. Carbohydr. Res. 338:2431–2447. 10.1016/j.carres.2003.07.010 [DOI] [PubMed] [Google Scholar]
- 25.Coderch N, Piqué N, Holst O, Merino S, Izquierdo L, Jimenez N, Tomás JM, Regué M. 2004. Serratia marcescens N28b (serovar O4) core lipopolysaccharide: structural and genetic characterization. J. Bacteriol. 186:978–988. 10.1128/JB.186.4.978-988.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Izquierdo L, Abitiu N, Coderch N, Hita B, Merino S, Gavin R, Tomás JM, Regué M. 2002. The inner-core lipopolysaccharide biosynthetic waaE gene: function and genetic distribution among some Enterobacteriaceae. Microbiology 148:3485–3496 [DOI] [PubMed] [Google Scholar]
- 27.Regué M, Izquierdo L, Fresno S, Piqué N, Corsaro MM, Naldi T, de Castro C, Waidelich D, Merino S, Tomás JM. 2005. A second outer-core region in the Klebsiella pneumoniae lipopolysaccharide. J. Bacteriol. 187:4198–4206. 10.1128/JB.187.12.4198-4206.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sambrook J, Fritsch EF, Maniatis T. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY [Google Scholar]
- 29.Sanger F, Nicklen S, Coulson AR. 1977. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. U. S. A. 74:5463–5467. 10.1073/pnas.74.12.5463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389–3402. 10.1093/nar/25.17.3389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thompson JD, Higgins D G, Gibson TJ. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673–4680. 10.1093/nar/22.22.4673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Darveau RP, Hancock REW. 1983. Procedure for isolation of bacterial lipopolysaccharides from both smooth and rough Pseudomonas aeruginosa and Salmonella typhimurium strains. J. Bacteriol. 155:831–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hitchcock PJ, Brown TM. 1983. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J. Bacteriol. 154:269–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Westphal O, Jann K. 1965. Bacterial lipopolysaccharide extraction with phenol-water and further application of the procedure. Methods Carbohydr. Chem. 5:83–89 [Google Scholar]
- 35.Galanos C, Lüderitz O, Westphal O. 1969. A new method for the extraction of R lipopolysaccharides. Eur. J. Biochem. 9:245–249. 10.1111/j.1432-1033.1969.tb00601.x [DOI] [PubMed] [Google Scholar]
- 36.Geerlof A, Lexendon A, Shaw V. 1999. Purification and characterization of phosphopantheine adenyltransferase from Escherichia coli. J. Biol. Chem. 274:27105–27111. 10.1074/jbc.274.38.27105 [DOI] [PubMed] [Google Scholar]
- 37.Piqué N, Aquilini E, Alioto T, Miñana-Galbisa D, Tomás JM. 2013. Genome sequence of Plesiomonas shigelloides strain 302-73 (serotype O1). Genome Announc. 1(4):e00404-13. 10.1128/genomeA.00404-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Izquierdo L, Coderch N, Piqué N, Bedini E, Corsaro MM, Merino S, Fresno S, Tomás JM, Regué M. 2003. The Klebsiella pneumoniae wabG gene: its role in the biosynthesis of the core lipopolysaccharide and virulence. J. Bacteriol. 185:7213–7221. 10.1128/JB.185.24.7213-7221.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Regué M, Izquierdo L, Fresno S, Jimenez N, Piqué N, Corsaro MM, Parrilli M, Naldi T, Merino S, Tomás JM. 2005. The incorporation of glucosamine into enterobacterial core lipopolysaccharide: two enzymatic steps are required. J. Biol. Chem. 280:36648–36656. 10.1074/jbc.M506278200 [DOI] [PubMed] [Google Scholar]
- 40.Fresno S, Jiménez N, Canals R, Merino S, Corsaro MM, Lanzetta R, Parrilli M, Pieretti G, Regué M, Tomás JM. 2007. A second galacturonic acid transferase is required for core lipopolysaccharide biosynthesis and complete capsule association to the cell surface in Klebsiella pneumoniae. J. Bacteriol. 189:1128–1137. 10.1128/JB.01489-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Amor K, Heinrichs DE, Frirdich E, Ziebell K, Johnson RP, Whitfield C. 2000. Distribution of core oligosaccharide types in lipopolysaccharides from Escherichia coli. Infect. Immun. 68:1116–1124. 10.1128/IAI.68.3.1116-1124.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aquilini E, Azevedo J, Merino S, Jiménez N, Tomás JM, Regué M. 2010. Three enzymatic steps required for the galactosamine incorporation into core lipopolysaccharide. J. Biol. Chem. 285:39739–39749. 10.1074/jbc.M110.168385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Salerno A, Delétoile M, Lefevre I, Ciznar I, Krovacek K, Grimont P, Brisse S. 2007. Recombining population structure of Plesiomonas shigelloides (Enterobacteriaceae) revealed by multilocus sequence typing. J. Bacteriol. 189:7808–7818. 10.1128/JB.00796-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hanahan D. 1983. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166:557–580. 10.1016/S0022-2836(83)80284-8 [DOI] [PubMed] [Google Scholar]
- 45.Belunis CJ, Clementz T, Carty SM, Raetz CHR. 1995. Inhibition of lipopolysaccharide biosynthesis and cell growth following inactivation of the kdtA gene of Escherichia coli. J. Biol. Chem. 270:27646–27652. 10.1074/jbc.270.46.27646 [DOI] [PubMed] [Google Scholar]
- 46.Guzman LM, Belin D, Carson MJ, Beckwith J. 1995. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J. Bacteriol. 177:4121–4130 [DOI] [PMC free article] [PubMed] [Google Scholar]