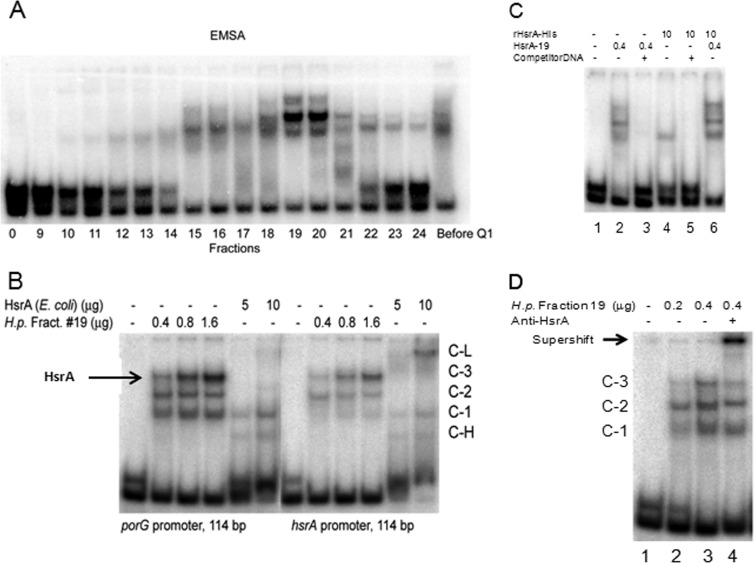
FIG 6.
Partial purification of proteins bound to the porG promoter. (A) Fractions eluted with an NaCl gradient from the UNO Q1 column (see Materials and Methods) were analyzed by EMSA. Note that in all EMSAs involving the porG amplicon, a control amplicon lacking HsrA binding sequences is included. (B) EMSA of porG and hsrA promoters in the presence of HsrA produced in E. coli (rHsrA-His10) or H. pylori (UNO Q1 column fraction 19). (C) EMSA of the porG promoter (114 bp) in the presence of competitor DNA (not-labeled hsrA promoter DNA; 114 bp; 70 ng). The amount of protein used for EMSA is depicted at the top. (D) Supershift with anti-HsrA serum and unbound DNA (lane 1). Protein concentrations (μg) and the presence or absence of anti-HsrA serum (lane 4) are indicated at the top of the gel. The supershift is indicated by an arrow. Note that the C-3 band in lane 4 was diminished compared to that in lane 3 and that the control amplicon had not shifted, a control for specificity.