FIG 3.
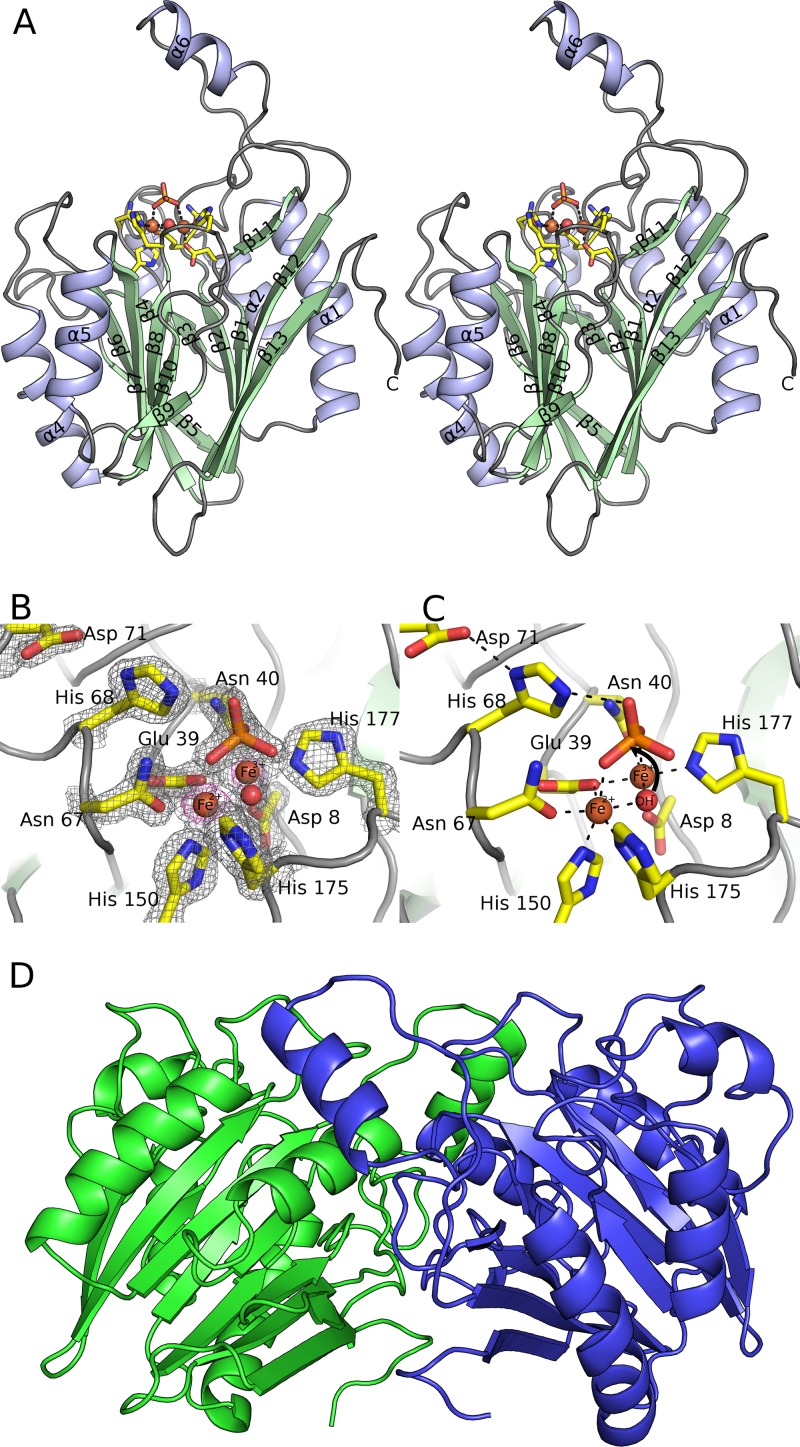
Crystal structure of YmdB. (A) Stereographic ribbon representation of the YmdB monomer, with α-helices in blue and β-sheets in green. Secondary structural elements are labeled according to topology. The active-site environment is also depicted, in stick mode colored by atom type, with bound ions and the proposed catalytic water as spheres. (B) The active site of YmdB, with key amino acids highlighted as sticks and colored by atom type. The final 2mFo-DFc electron density map is contoured at a level of 1.9 σ and is shown as a gray mesh. An anomalous-difference Fourier series, using phases calculated from the refined model, is contoured at a level of 5 σ (pink). The only peaks of this height in the Fourier series correspond to the two Fe ions at the active site. (C) The active site of YmdB in the same orientation as that in panel B, with key amino acids highlighted as sticks and colored by atom type. Hydrogen bonds are shown as dashed black lines. (D) The YmdB dimer, with each chain colored independently. Helix α6 from chain A (blue) packs above the region surrounding strands β9 and β11 in chain B (green).