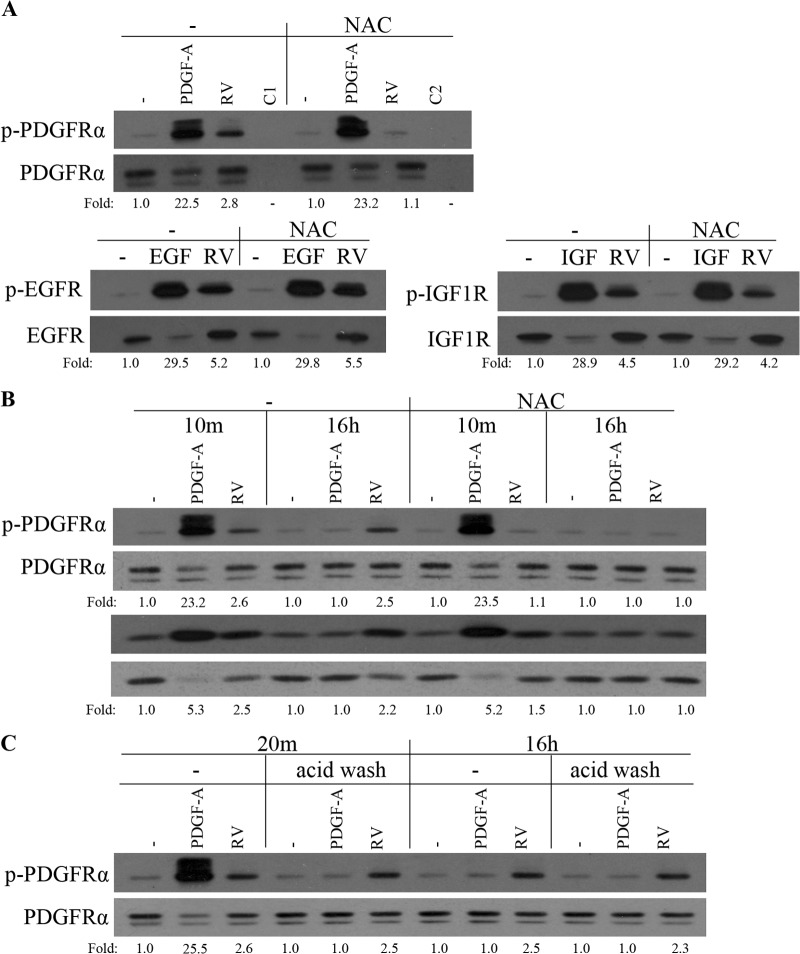
FIG 3.
Vitreous-driven activation of PDGFRα was selective, prolonged, and dependent on ROS. (A) Serum-starved ARPE19α cells were pretreated with NAC (10 mM) for 30 min and then stimulated with buffer (−), with PDGF-A, EGF, or IGF-1 (IGF) (each at 50 ng/ml), or with RV for 10 min. The lanes designated C1 and C2 were stimulated with buffer and RV, respectively. The cells were lysed, and the resulting lysates were immunoprecipitated using antibodies against PDGFRα, EGFR, IGF-1R, or nonimmune IgG (C1 and C2). The resulting immunoprecipitates were subjected to Western blot analysis using an antiphosphotyrosine antibody (pY20). The membranes were reprobed with antibodies against PDGFRα, EGFR, or IGF-1R. The fold values are the p-PDGFRα/PDGFRα ratio, the p-EGFR/EGFR ratio, or the p-IGF-1R/IGF-1R ratio. The data are representative of three experiments; similar results were obtained when the experiment was repeated with primary human corneal fibroblasts instead of ARPE19α cells (data not shown). (B) Serum-deprived ARPE19α cells were pretreated with NAC (10 mM) for 30 min before stimulation with PDGF-A (50 ng/ml) or RV for 10 min. For the 16-h stimulation with PDGF-A or RV, the 30-min NAC (10 mM) treatment was from 15.5 to 16 h. Following stimulation, the cells were lysed and subjected to Western blotting using the indicated antibodies. The fold values are the p-PDGFRα/PDGFRα ratio or the p-Akt/Akt ratio. The blots presented are representative of three experiments. (C) Serum-starved ARPE19α cells were treated with PDGF-A (50 ng/ml) or RV for 10 min, acid washed (to remove the growth factors), and harvested either at 10 min or at 15 h and 50 min later. The lysates were subjected to Western blot analysis using a phospho-PDGFRα Y754 antibody. The membrane was reprobed with a PDGFRα antibody (27P). The fold values are the p-PDGFRα/PDGFRα ratio. The data presented are representative of three experiments.