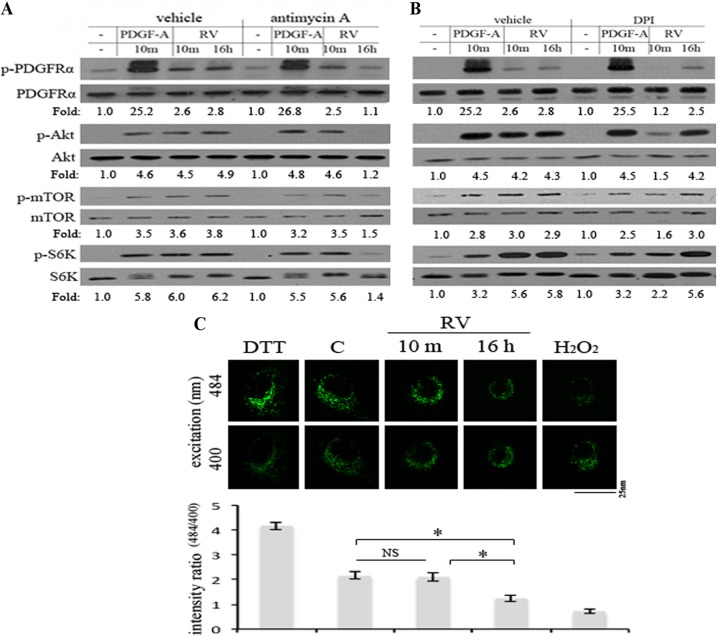
FIG 7.
Mitochondrial ROS was required for persistent activation of PDGFRα in response to RV. (A and B) Near-confluent, serum-starved ARPE19α cells were pretreated with antimycin A (0.5 μM) (a mitochondrial electron transport inhibitor) or diphenyleneiodonium chloride (DPI) (5 μM) (an NADPH oxidase [NOX] inhibitor) for 20 min, after which time RV was added and the cells were lysed 10 min later; the cells were exposed to drug and RV for 30 and 10 min, respectively. To monitor the effects of the inhibitors at the 16-h time point, the cells were first exposed to RV for 15.5 h, the inhibitors were added, and the cells were lysed 30 min later; the cells were exposed to drug and RV for 30 min and 16 h, respectively. The lysates were subjected to Western blot analyses using the indicated antibodies. The fold values are the p-PDGFRα/PDGFRα ratio, the p-Akt/Akt ratio, the p-mTOR/mTOR ratio, or the p-S6K/S6K ratio. The data presented are representative of three independent experiments. Similar results were observed when carbonyl cyanide 3-chlorophenylhydrazone (CCCP) (50 μM) (a mitochondrial electron transport inhibitor) and acetovanillone (apocynin) (10 μM) (a NOX inhibitor) were used instead of antimycin and DPI (data not shown). (C) Near-confluent, serum-starved ARPE19α cells stably expressing a mitochondrion-localized redox-sensitive GFP mutant (31) were treated with DTT (1 mM) for 1 h, RV for 10 min or 16 h, or H2O2 (1 mM) for 30 min. The panels are photos of representative cells that were illuminated with an excitation wavelength of 400 nm or 484 nm and an emission wavelength of 525 nm. Five exposures were measured of different areas (consisting of the three to five cells) for each experimental condition. Values in the bar graph are means ± SD from at least 3 independent experiments. Values that are statistically significantly different (P < 0.05) are indicated by an asterisk and bar. Values that are not statistically significantly different are indicated by NS and a bar.