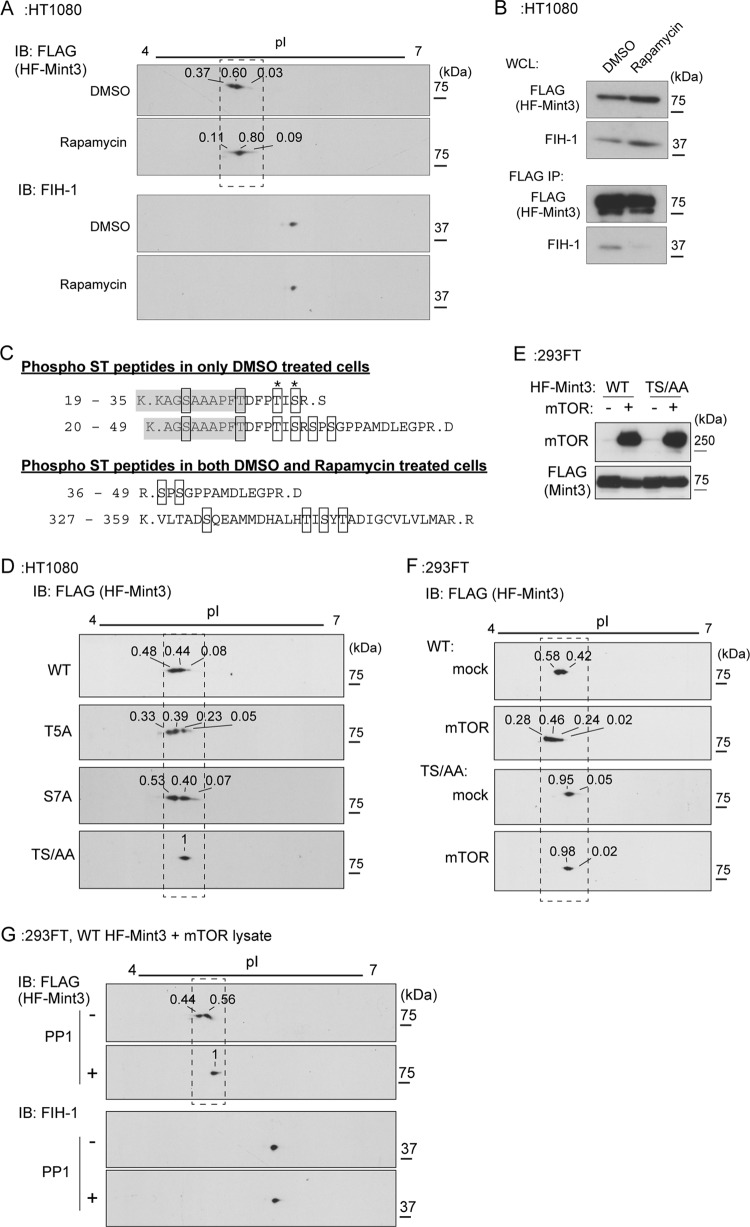
FIG 4.
Mint3 is phosphorylated by mTOR. (A) Rapamycin treatment increased the isoelectric point of the HF-Mint3 protein. IB, immunoblot. (B) Rapamycin treatment decreased the binding of Mint3 to FIH-1. His6- and FLAG-tagged Mint3 (HF-Mint3) in vehicle- or rapamycin-treated HT1080 cells was precipitated with anti-FLAG antibody beads, and the eluate was subjected to immunoblot analysis. WCL, whole-cell lysates. (C) Phosphorylated peptides of HF-Mint3 were identified by mass spectrometry. The shaded amino acids were derived from the linker sequence between the His6 tag and Mint3. DMSO, dimethyl sulfoxide. (D) TS/AA mutant Mint3 migrated in the form of a single polypeptide in 2D electrophoresis. (E and F) The expression of mTOR decreased the isoelectric point of WT but not TS/AA mutant HF-Mint3 in 293FT cells. (E) Expression of mTOR and WT or TS/AA mutant Mint3 in 293FT cells was confirmed by immunoblot analysis. (F) 2D electrophoresis and immunoblot analysis were performed with the cell lysates assessed in panel E. (G) Phosphatase treatment increased the isoelectric point of WT HF-Mint3 coexpressed with mTOR. In panels A, D, F, and G, the relative intensity of each HF-Mint3 signal is shown above the spot. For enlargements of the boxed areas and a densitometry analysis, see Fig. S3 in the supplemental material.