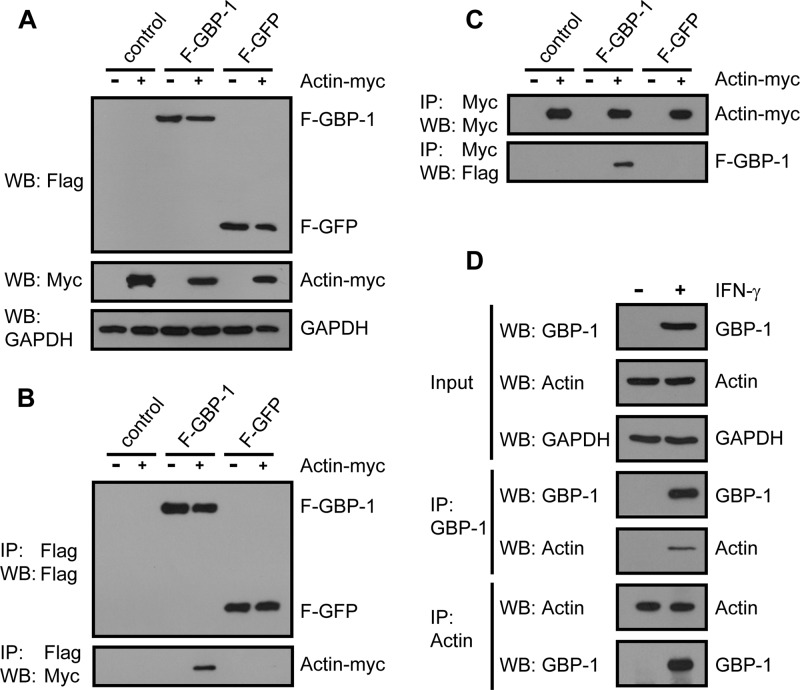
FIG 2.
Interaction of tagged and endogenous GBP-1 and actin proteins shown by reciprocal coimmunoprecipitation. (A) HeLa cells were cotransfected with F–GBP-1, F-GFP, or the empty parental vector each together with an expression plasmid encoding Myc-tagged actin (actin-Myc) or the corresponding control vector. Subsequently, 10 μg of lysate was analyzed via Western blotting (WB) using antibodies against the Flag and Myc tags. GAPDH was used as a loading control. (B) Immunoprecipitation analysis of the extracts from panel A with anti-Flag antibody and subsequent Western blot analysis with antibodies against either the Flag or Myc tag. (C) Lysates, transfected and analyzed as described in the legend to panel A, were immunoprecipitated using an antibody against the Myc tag. The precipitated proteins were subsequently analyzed via Western blotting using the indicated antibodies. (D) HeLa cells were either left untreated or treated with IFN-γ (100 U/ml, 24 h). The protein extracts were immunoprecipitated using an antibody against GBP-1 or actin and analyzed via Western blotting. GBP-1 and actin were detected using specific antibodies.