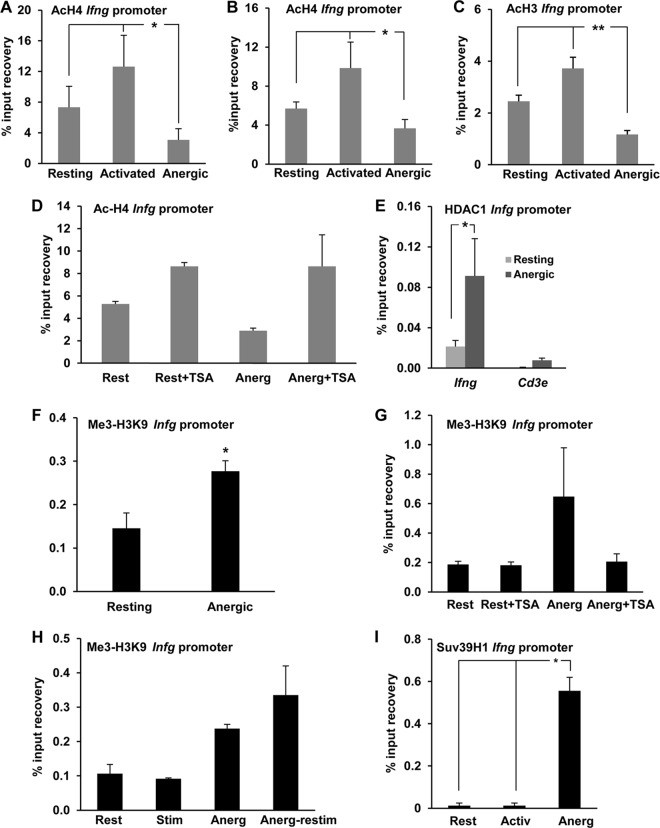
FIG 2.
Anergizing stimuli induce histone deacetylation and H3K9 trimethylation at the Ifng promoter. (A to C) TH1 cells were anergized either with ionomycin (A) or through stimulation with anti-CD3 antibody in the absence of costimulation (B and C) for 16 h. Cells were then collected and analyzed for H4 (A and B) or H3 (C) acetylation at the Ifng promoter by ChIP using an anti-acetyl H4 (AcH4) or H3 (AcH3) antibody. Controls included resting cells and cells activated through stimulation with anti-CD3 and anti-CD28 antibodies. Results show means and SEM for 3, 4, and 5 independent experiments for panels A, B, and C, respectively. *, P < 0.05; **, P < 0.01. (D) ChIP assays were carried out with AcH4 antibody on resting (Rest) and anergized (Anerg) TH1 cells in the presence or absence of the histone deacetylase inhibitor TSA. Results show means and SEM for 4 independent experiments. (E) ChIP assays were performed on resting and anergized TH1 cells with an anti-HDAC1 antibody to assess HDAC1 occupancy of the Ifng promoter. The Cd3e promoter was used as a control. Results show means and SEM for 3 independent experiments. *, P < 0.05. (F and G) TH1 cells were treated as described for panels B and D, respectively, and H3K9 trimethylation (Me3-H3K9) at the Ifng promoter was assessed by ChIP. Results show means and SEM for 4 independent experiments. *, P < 0.05. (H) Control and anergized (Anerg) TH1 cells were either kept resting (Rest) or restimulated with anti-CD3 and anti-CD28 antibodies (Stim). The Ifng promoter was probed for the Me3-H3K9 histone modification mark by ChIP. Results are means and SEM for 3 independent experiments. (I) Resting, activated (Activ), and anergized (Anerg) TH1 cells were analyzed by ChIP using an antibody specific for the histone methyltransferase Suv39H1 to assess occupancy of the Ifng promoter by Suv39H1. Results show means and SEM for 3 independent experiments. *, P < 0.05.