Abstract
Ureaplasma urealyticum is a fastidious bacterium usually residing in the female genitourinary tract. We present an exceedingly complicated case of a brain abscess secondary to mastoiditis by U. urealyticum in an adult hypogammaglobulinemic patient after rituximab treatment 3 years earlier.
CASE REPORT
On 11 February 2013, a 20-year-old patient presented to our ear, nose, and throat (ENT) clinics with a 3-week history of putrid discharge from the left ear, otalgia, and a left-side paresis of the facial nerve.
The past medical history was notable for Burkitt's lymphoma, diagnosed in October 2009. Initial staging showed Ann Arbor stage IV BE, International Prognostic Index of 4, with tumor involvement of the anterior mediastinum, intercostal, and pectoralis muscles, a large intraperitoneal mass, and pleurosis lymphomatosa. No bone marrow involvement could be demonstrated by biopsy, and the patient was human immunodeficiency virus (HIV) negative. The patient was treated according to the German B-NHL/B-ALL protocol, involving several cycles of combination chemotherapy, including vincristine, methotrexate, ifosfamide, etoposide, cytarabine, cyclophosphamide, doxorubicin (Adriamycin), vindesine, dexamethasone, and rituximab. Therapy was well tolerated, with one occurrence each of orofacial herpes simplex virus (HSV) reactivation and mucosal candidiasis. The patient had been in complete remission since April 2010 with no signs of relapse at the time of the current complaints. During routine follow-up examinations, no signs of any unusual infections were noted.
Clinical examination revealed an edematous left-side external auditory canal, with incomplete visualization of the tympanic membrane. Audiometry showed left-side sensineural and conductive hearing loss. Laboratory investigations were notable for mild leukocytosis (leukocytes, 14.7/nl [normal, 3 to 10/nl]; 83% neutrophils; 2% band forms; 1% metamyelocytes), thrombocytosis (thrombocytes, 460/nl [normal, 140 to 440/nl]), and elevated serum C-reactive protein (11.9 mg/dl; normal, <0.5 mg/dl). Other laboratory values were within normal limits.
A computed tomography (CT) scan performed on 11 February demonstrated opacity of the entire left-side mastoid cells, the tympanum, and the external auditory canal. Furthermore, a small osseous defect between the mastoidal cells and the middle cranial fossa on coronary reconstructions, without demonstration of intracerebral abscess formation, was suspected. Moreover, hypodensity of the left-side sigmoid sinus raised a high suspicion of sinus venous thrombosis. This could be confirmed by a CT angiogram performed on 12 February, which demonstrated complete occlusion of the left-side sigmoid and transverse sinus.
On 12 February, a subtotal mastoidectomy with drainage of the tympanum and opening of the left sigmoid sinus was performed, with one revision operation performed on 18 February. Several swab cultures (blood, chocolate, MacConkey, Schaedler and Schaedler, and kanamycin/vancomycin agar plates plus thioglycolate broth, all incubated under the proper aerobic or anaerobic conditions at 37°C for at least 48 h) submitted for routine microbiological examination remained sterile. The patient was placed on intravenous (i.v.) cefuroxime and metronidazole, and systemic anticoagulation with heparin was started. Symptoms improved postoperatively, and facial nerve paresis resolved, so the patient was placed on oral clindamycin on 20 February. A control magnetic resonance tomography (MRT) procedure performed on 27 February showed, besides the obvious postoperative osseous defect (with the remaining mastoid cells still being opaque), a white-matter edema in the left occipital lobe, which was at that time interpreted as secondary to the venous congestion caused by the sinus thrombosis (Fig. 1). The patient was discharged home on 5 March.
FIG 1.
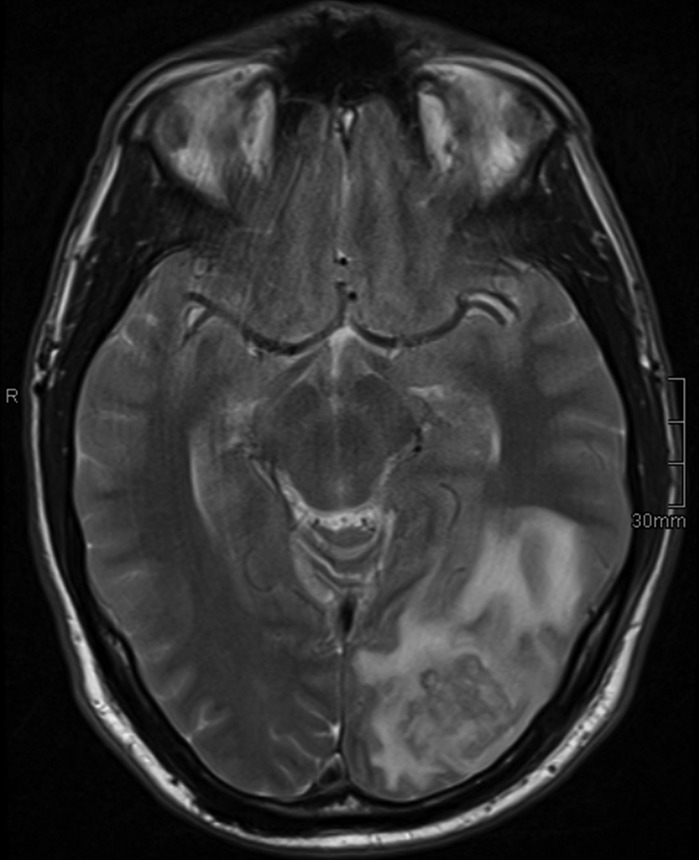
MRT performed on 27 February (T2-weighted image). Note left occipital white-matter edema, which was interpreted as secondary to venous occlusion.
On 7 March, a scheduled control MRT showed a massive enlargement of the occipital process (Fig. 2), causing a 3-mm midline shift, which was now interpreted as representing intracerebral abscess formation. Moreover, the thrombosis of the left transverse sinus progressed. At that time, the patient suffered from homonymous hemianopia to the right and motor aphasia. He was scheduled for abscess drainage and thrombectomy on 8 March and was placed on i.v. meropenem. Since the patient progressed significantly under standard treatment and the thrombus was expected to be of considerable size and volume, intravascular recanalization was performed. Abscess contents were sent for routine microbiological examination and remained sterile even upon prolonged incubation for 14 days. A pathological examination showed no evidence of malignancy, ruling out a relapse of the Burkitt's lymphoma. The serum procalcitonin level on 8 March was 0.49 ng/ml.
FIG 2.
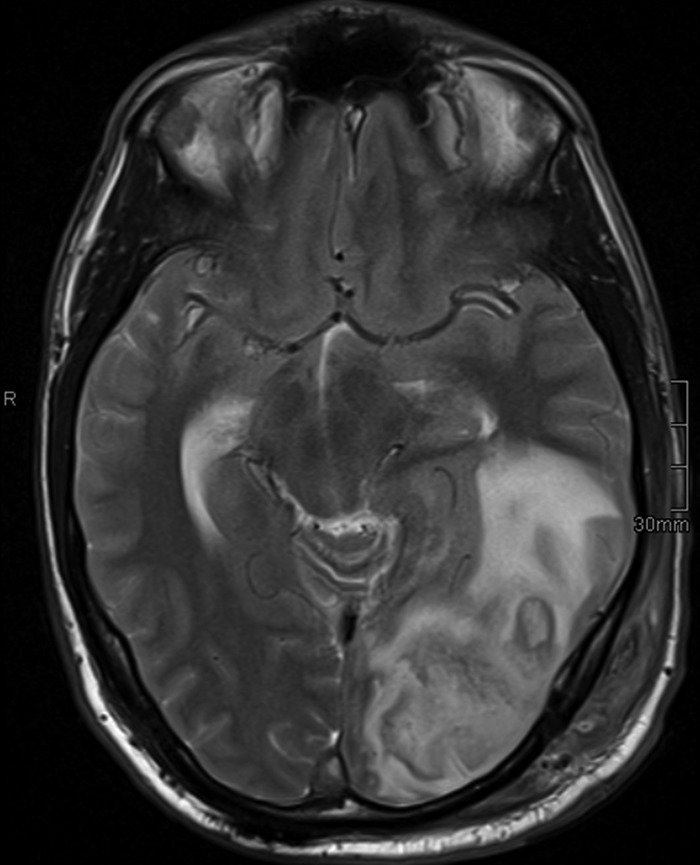
MRT performed on 7 March (T2-weighted image), demonstrating massive increase of the left occipital lesion with intracerebral abscess formation.
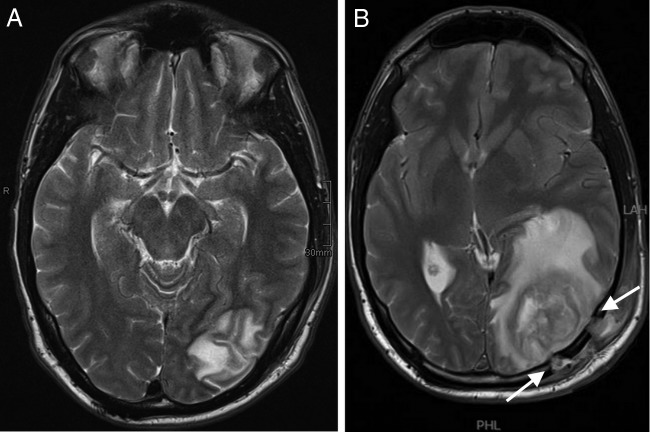
A beta trace examination performed on 13 March confirmed the wound discharge fluid to be cerebrospinal fluid (CSF). Another swab culture from the operation site remained sterile. A control MRT performed on 15 March again showed substantial progression of the abscess, with pus draining from the trepanation holes into the subgaleal soft tissue, and a midline shift of 12 mm (Fig. 3). Moreover, sinus thrombosis also progressed despite adequate anticoagulation, now encompassing also the right-side sigmoid and transverse sinus. Another abscess resection and revision of the mastoid cells were performed on 16 March. The patient was placed on intravenous meropenem, vancomycin, and metronidazole (the latter being discontinued after routine microbiologic counseling on 17 March) and was switched to intravenous meropenem plus linezolid on 18 March.
FIG 3.
(A) MRT performed on 15 March (T2-weighted image), showing further increases in the size of the abscesses and the midline shift. (B) Note pus draining through the trepanation holes into the subgaleal tissue (arrows).
On 19 March, serum immunoglobulin levels were examined, and very low levels of all Ig classes could be detected: IgA at 25.2 mg/dl (normal, 70 to 400 mg/dl), IgG at 210 mg/dl (normal, 700 to 1,600 mg/dl), and IgM at undetectable levels with <5.3 mg/dl (normal, 40 to 230 mg/dl). Since several thrombectomy attempts had failed and cerebral edema progressed significantly, a decision was made by our interventional neuroradiology department to perform stenting of the entire sigmoid and transverse sinus down to the bulbus of the internal jugular vein as a “compassionate use” intervention. However, only after percutaneous transluminal angioplasty could a sufficient venous flow be established.
Material from the 16 March operation was sent in for microbiology and, again, remained sterile in routine cultures. However, one sample was sent for broad-range 16S rRNA gene PCR. On 20 March, sequencing of a PCR amplicon revealed the presence of Ureaplasma urealyticum DNA (99% sequence homology to the 16S rRNA gene sequence of strain ATCC 33699 and several other strains). This could subsequently be confirmed by a pathogen-specific PCR assay discriminating between U. urealyticum and U. parvum, ruling out U. parvum as the causative agent (1). Fortunately, an aliquot of the clinical sample was retained in the laboratory and streaked on Ureaplasma agar (Oxoid). After anaerobic incubation overnight, growth of urea-utilizing colonies with typical (granular cotton-wool-like) microscopic morphology for U. urealyticum could be detected.
The patient was immediately switched to high-dose intravenous doxycycline (200 mg i.v. twice a day [b.i.d.]) and clarithromycin (500 mg i.v. b.i.d.) on 20 March, and i.v. immunoglobulins were administered. He subsequently improved significantly and was discharged from the intensive care unit (ICU) on 23 March. Serum immunoglobulin levels were nearly normal on 25 March. Neutrophils were normal for the first time on 30 March. A control MRT on 22 March and a control CT scan on April 4 demonstrated regressing size of the abscess and normalization of the midline shift. On 5 April, immunophenotyping of peripheral blood leukocytes confirmed the complete absence of B lymphocytes. The patient was discharged to a neurological rehabilitation facility on 9 April.
Ureaplasma urealyticum is fastidious, primarily utilizes urea to generate ATP, and lacks a cell wall. It can be found in the vaginal flora in a high percentage of asymptomatic women, and its presence has been linked to infertility and adverse pregnancy outcomes. It causes a variety of diseases in newborns, particularly in preterm infants, including congenital pneumonia, bronchopulmonary dysplasia, intraventricular hemorrhage, retinopathy of prematurity, and others (2).
Extragenital infections in adults are rare. Occasionally, postoperative infections have been described: Lucke et al. reported the case of a patient with a deep sternal wound infection after aortic valve replacement (3). García-de-la-Fuente et al. described a patient with postoperative mediastinitis, pleuritis, and pericarditis caused by U. urealyticum after replacement of the aortic valve and ascending aorta, ultimately leading to septic shock, multiorgan failure, and death (4). Interestingly, both patients had no apparent risk factors (e.g., immunosuppression) that could help explaining the unusual severe course of these infections.
Hypogammaglobulinemia seems to be a risk factor for invasive infections with Ureaplasma or Mycoplasma species. Most often, these manifest as septic arthritis (5) but may also present as disseminated infections. Endocarditis and osteomyelitis caused by U. urealyticum was described in a patient with common variable immunodeficiency (CVID) (6). Another CVID patient suffered from disseminated Ureaplasma infection involving the skin, subcutaneous tissue, CSF, kidney, a pelvic abscess, and sepsis but survived after pathogen-specific antimicrobial therapy (7). Similar cases have been reported after rituximab therapy. Septic arthritis was described in a patient treated for systemic lupus erythematodes with rituximab (8). A second patient, who was on rituximab maintenance therapy for non-Hodgkin's lymphoma, developed septic arthritis from Mycoplasma hominis and Ureaplasma parvum which disseminated into a mycotic aortic aneurysm. The patient required two replacements of the abdominal aorta and ultimately developed fatal septic shock (9). Finally, another patient experienced septic U. urealyticum polyarthritis after rituximab-containing treatment for diffuse large B cell lymphoma, 8 months post-hematopoietic stem cell transplantation (HSCT), still being agammaglobulinemic (10).
Although some of these patients had clinical signs of central nervous system (CNS) involvement, overt CNS infection is extremely rare. To the best of our knowledge, only two cases have been described in the literature. One patient developed U. urealyticum meningitis after kidney transplantation, which was complicated by arterial bleeding, mass transfusions, infections of a retroperitoneal hematoma, and intraperitoneal abscess formation by classical nosocomial pathogens (Pseudomonas aeruginosa, Enterococcus faecium, ESBL-positive Morganella morganii, Klebsiella pneumonia, and Candida albicans) (11). Unfortunately, no information on serum immunoglobulin levels from this patient is available. A second report is available which describes a brain abscess in a previously healthy full-term newborn; Mycoplasma hominis and Ureaplasma spp. (not further differentiated) were detected (12). Again, no information on serum immunoglobulin levels is available from the case report.
Our case—the first adult U. urealyticum brain abscess described in the medical literature—shows that in a select group of patients with hypogammaglobulinemia, Ureaplasma should be considered etiologic agents in intracerebral infections. However, the diagnosis is still challenging. The paucity of CNS infections caused by Mycoplasma or Ureaplasma species probably does not justify routine culture on specialized media. Should we then perform molecular tests, by broad-range PCR and sequencing, in all cases of intracerebral infections? In about one-fourth of cases, this strategy is also bound to fail, since multiple organisms may be present (13). Subcloning of PCR amplicons with subsequent sequencing of individual clones is beyond the possibilities of most clinical microbiology laboratories. An effective strategy may be to retain a portion of the brain abscess sample in the laboratory and perform molecular diagnostics after incubation for 48 h if the incubation fails to yield results (14). If we had employed this strategy, we could have identified the etiologic agent 6 days earlier than we actually did and could probably have avoided the second craniotomy.
This case also illustrates the power of rituximab to suppress B cell development in younger patients. Our patient had received the last dose of rituximab maintenance therapy on 10 May 2010—3 years before presenting with the current complaints. Still, no B cells could be detected by immunophenotyping in peripheral blood, and hypogammaglobulinemia was profound, when the patient presented to us. It should be noted that during the course of infection, no apparent signs of any genitourinary infections were apparent; however, a formal examination and an attempt to culture U. urealyticum have not been performed. Thus, the source of the infection—as in most other cases—remains unclear.
The optimal treatment of severe or disseminated Ureaplasma infections is poorly defined. Several regimes have been suggested, mostly containing doxycycline plus a modern macrolide. Occasionally, quinolones, clindamycin, or chloramphenicol is also included. It should be noted that some beneficial clinical effect may be obtained with treatment not specific for Ureaplasma (7), reducing the suspicion of the treating clinician with respect to atypical agents of infection. A standardized method for antibiotic susceptibility testing of Ureaplasma and mycoplasmas has recently been described (15). However, there are still substantial differences in measured MIC values between laboratories, even for defined QC strains. This is particularly true for azithromycin and tetracycline (15). Clinical MIC breakpoints have not yet been defined. In a recent evaluation of 63 clinical isolates, however, doxycycline and clarithromycin had the lowest MIC values of all agents tested, indicating that they can generally be considered active against U. urealyticum (16). In our institution, we do not have systems for susceptibility testing of ureaplasmas; therefore, we chose to treat the patient empirically with doxycycline plus clarithromycin, with prompt success.
Footnotes
Published ahead of print 11 December 2013
REFERENCES
- 1.Yi J, Yoon BH, Kim EC. 2005. Detection and biovar discrimination of Ureaplasma urealyticum by real-time PCR. Mol. Cell. Probes 19:255–260. 10.1016/j.mcp.2005.04.002 [DOI] [PubMed] [Google Scholar]
- 2.Viscardi RM. 19 August 2013. Ureaplasma species: role in neonatal morbidities and outcomes. Arch. Dis. Child. Fetal Neonatal Ed. 10.1136/archdischild-2012-303351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lucke K, Kuster SP, Bertea M, Ruef C, Bloemberg GV. 2010. A deep sternal wound infection caused by Ureaplasma urealyticum. J. Med. Microbiol. 59:1254–1256. 10.1099/jmm.0.022814-0 [DOI] [PubMed] [Google Scholar]
- 4.García-de-la-Fuente C, Miñambres E, Ugalde E, Sáez A, Martinez-Martinez L, Fariñas MC. 2008. Post-operative mediastinitis, pleuritis and pericarditis due to Mycoplasma hominis and Ureaplasma urealyticum with a fatal outcome. J. Med. Microbiol. 57:656–657. 10.1099/jmm.0.47632-0 [DOI] [PubMed] [Google Scholar]
- 5.Franz A, Webster AD, Furr PM, Taylor-Robinson D. 1997. Mycoplasmal arthritis in patients with primary immunoglobulin deficiency: clinical features and outcome in 18 patients. Br. J. Rheumatol. 36:661–668. 10.1093/rheumatology/36.6.661 [DOI] [PubMed] [Google Scholar]
- 6.Frangogiannis NG, Cate TR. 1998. Endocarditis and Ureaplasma urealyticum osteomyelitis in a hypogammaglobulinemic patient. A case report and review of the literature. J. Infect. 37:181–184 [DOI] [PubMed] [Google Scholar]
- 7.Cordtz J, Jensen JS. 2006. Disseminated Ureaplasma urealyticum infection in a hypo-gammaglobulinaemic renal transplant patient. Scand. J. Infect. Dis. 38:1114–1117. 10.1080/00365540600675734 [DOI] [PubMed] [Google Scholar]
- 8.Goulenok TM, Bialek S, Gaudart S, Bebear C, Fantin B. 2011. Ureaplasma urealyticum destructive septic arthritis in a patient with systemic lupus erythematosus after rituximab therapy. Joint Bone Spine 78:323–324. 10.1016/j.jbspin.2010.11.010 [DOI] [PubMed] [Google Scholar]
- 9.MacKenzie CR, Nischik N, Kram R, Krauspe R, Jager M, Henrich B. 2010. Fatal outcome of a disseminated dual infection with drug-resistant Mycoplasma hominis and Ureaplasma parvum originating from a septic arthritis in an immunocompromised patient. Int. J. Infect. Dis. 14(Suppl 3):e307–e309. 10.1016/j.ijid.2010.02.2253 [DOI] [PubMed] [Google Scholar]
- 10.Arber C, Buser A, Heim D, Weisser M, Tyndall A, Tichelli A, Passweg J, Gratwohl A. 2007. Septic polyarthritis with Ureaplasma urealyticum in a patient with prolonged agammaglobulinemia and B-cell aplasia after allogeneic HSCT and rituximab pretreatment. Bone Marrow Transplant. 40:597–598. 10.1038/sj.bmt.1705766 [DOI] [PubMed] [Google Scholar]
- 11.Geissdörfer W, Sandner G, John S, Gessner A, Schoerner C, Schröppel K. 2008. Ureaplasma urealyticum meningitis in an adult patient. J. Clin. Microbiol. 46:1141–1143. 10.1128/JCM.01628-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rao RP, Ghanayem NS, Kaufman BA, Kehl KS, Gregg DC, Chusid MJ. 2002. Mycoplasma hominis and Ureaplasma species brain abscess in a neonate. Pediatr. Infect. Dis. J. 21:1083–1085. 10.1097/00006454-200211000-00026 [DOI] [PubMed] [Google Scholar]
- 13.Tseng JH, Tseng MY. 2006. Brain abscess in 142 patients: factors influencing outcome and mortality. Surg. Neurol. 65:557–562; discussion 562. 10.1016/j.surneu.2005.09.029 [DOI] [PubMed] [Google Scholar]
- 14.Petti CA, Simmon KE, Bender J, Blaschke A, Webster KA, Conneely MF, Schreckenberger PC, Origitano TC, Challapalli M. 2008. Culture-negative intracerebral abscesses in children and adolescents from Streptococcus anginosus group infection: a case series. Clin. Infect. Dis. 46:1578–1580. 10.1086/587655 [DOI] [PubMed] [Google Scholar]
- 15.Waites KB, Duffy LB, Bebear CM, Matlow A, Talkington DF, Kenny GE, Totten PA, Bade DJ, Zheng X, Davidson MK, Shortridge VD, Watts JL, Brown SD. 2012. Standardized methods and quality control limits for agar and broth microdilution susceptibility testing of Mycoplasma pneumoniae, Mycoplasma hominis, and Ureaplasma urealyticum. J. Clin. Microbiol. 50:3542–3547. 10.1128/JCM.01439-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Samra Z, Rosenberg S, Dan M. 2011. Susceptibility of Ureaplasma urealyticum to tetracycline, doxycycline, erythromycin, roxithromycin, clarithromycin, azithromycin, levofloxacin and moxifloxacin. J. Chemother. 23:77–79 [DOI] [PubMed] [Google Scholar]