Abstract
Use of a new collection kit for vaginal and cervical sampling was reported as easy by the majority of 692 women and not uncomfortable (by 87.4% of those ≥25 years old and 78.8% of those <25 years old). By Aptima testing, patient- and physician-collected samples agreed strongly for Chlamydia trachomatis (99.6% to 99.3%; κ = 0.93 to 0.89) and T. vaginalis (99.6% to 98.9%; κ = 0.97 to 0.78).
TEXT
Chlamydia trachomatis and Trichomonas vaginalis are common sexually transmitted infections (STI) of the lower genital tract. Control programs require screening to detect and treat asymptomatic infections to prevent upper tract complications due to C. trachomatis and persistent T. vaginalis infections and related sequelae.
Detection of infections requires the collection of endocervical or vaginal samples during a pelvic examination or self-collection of first-void urine or vaginal samples. If self-swabbing is to be used for screening, the procedure and collection device will be more acceptable if the device is easy to use and is not uncomfortable.
A new specimen collection and transportation (SCT) kit (Hologic/Gen-Probe Incorporated) was developed for sampling the cervix and vagina for STIs and transporting the specimens to the laboratory to be tested by Aptima transcription-mediated amplification assays. The collection device is a tapered brush similar in appearance to collection brushes used for collecting cervical cells for human papillomavirus testing (1, 2). The objective was to determine the ease and comfort of using the SCT when women self-collected a vaginal sample and to compare the new SCT kit for detection of C. trachomatis and T. vaginalis from self-collected vaginal SCT (S-VSCT) and physician-collected vaginal and cervical samples.
A total of 708 women (580 attending a gynecology clinic and 128 attending a street youth health clinic) signed consent for the collection of 2 vaginal and 3 cervical samples as outlined in the consent form, which was approved by St. Joseph's Healthcare and Juravinski Hospital Research Ethics Boards in Hamilton, Ontario, Canada. Each patient self-collected a vaginal sample and then answered a questionnaire concerning ease and comfort of collection using the new SCT kit. The physician then collected a vaginal sample (P-VSCT) and, after insertion of a speculum, collected PreservCyt (PC) L-Pap, cervical SCT (CSCT), and SurePath (SP) L-Pap endocervical samples. The PC L-Pap sample was always collected first as the standard of care for cervical cytology. L-Pap samples were collected with a cervix broom placed into the manufacturer's medium. The PC L-Pap sample was processed for cytology, and the remainder of the sample was sent with the other samples to the Infections Research Laboratory (IRL) at St. Joseph's Healthcare, where they were tested within 72 h by Aptima Combo 2 (AC2) for C. trachomatis and Aptima T. vaginalis (ATV) for T. vaginalis on a TIGRIS DTS instrument (Hologic/Gen-Probe Incorporated).
Each patient was asked to complete a 5-point Likert scale questionnaire indicating whether it was very easy, easy, neither easy nor difficult, difficult, or very difficult to open the package and take out the tube and collection device, collect the sample, uncap the tube, elute the sample, and recap the tube. They were also asked whether collection was comfortable or uncomfortable.
Agreement between sample types was assessed as raw agreement and as agreement beyond chance (using the kappa statistic [κ]) along with 95% confidence intervals.
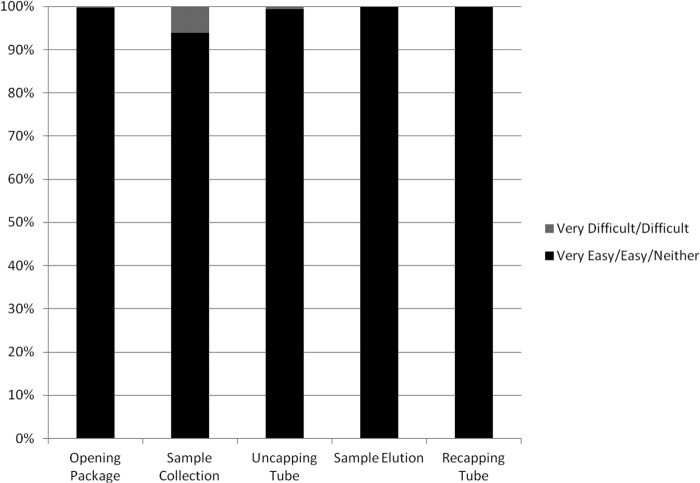
The responses to the ease and comfort of collection with the new SCT kit were very favorable (Fig. 1). The survey was completed by 692 women. One of the strengths of the study was the number of women from 2 different clinics who were able to assess the ease and comfort of self-collecting vaginal samples with the new SCT kit. Although almost all reported that opening the package (99.7%), collecting the sample (93.9%), uncapping the tube (99.3%), eluting the sample from the brush (99.9%), and recapping the tube (99.7%) were very easy, easy, or neither easy nor difficult, 6.1% experienced some difficulty in self-collecting the sample, and this was not related to age. Collection was not uncomfortable for 84.3% of the women, and a subanalysis of the question according to age showed that 87.4% of women 25 years or older (n = 480) and 78.8% of those <25 years of age (n = 212) reported that the collection process was not uncomfortable (P = 0.005). These observations are similar to other studies assessing self-collection of vaginal samples using swabs (3–6). Other studies have reported that self-collection of vaginal samples was not preferred over collection by health care workers (7–10) but was acceptable. The reasons for not preferring self-collection of vaginal samples have included concerns about collecting an inadequate sample (10–13) and that self-collection was not as comfortable as collection by health care workers (12).
FIG 1.
Summary of ease of self-collecting vaginal samples using a new specimen collection and transportation (SCT) kit.
Prevalences of C. trachomatis and T. vaginalis infections were 12.5% and 13.4% in the youth health clinic and 1.0% and 0.3% in the gynecology clinic. The prevalences of C. trachomatis and T. vaginalis in our younger sexually active women are proportionately similar to 8.7% and 6.7%, respectively, reported in a previous study using the Aptima assay (14).
Strong overall agreements of C. trachomatis positives and negatives were observed between S-VSCT and the physician-collected P-VSCT (99.6%; κ = 0.93), CSCT (99.4%; κ = 0.91), PC L-Pap (99.4%; κ = 0.91), and SP L-Pap (99.3%; κ = 0.88) samples (Table 1). Similarly, strong overall agreements were calculated for T. vaginalis between self-collected vaginal samples and physician-collected vaginal and cervical samples: S-VSCT to P-VSCT (99.9%; κ = 0.97), CSCT (99.7%; κ = 0.94), PC L-Pap (99.6%; κ = 0.91), and SP L-Pap (98.8%; κ = 0.78) (Table 2).
TABLE 1.
Overall agreement of S-VSCT samples with P-VSCT, CSCT, PC, and SP samples for C. trachomatisa
| Sample | Result | No. of S-VSCT samples |
% overall agreement (95% CI) | Kappa (95% CI) | ||
|---|---|---|---|---|---|---|
| + | − | Total | ||||
| P-VSCT | + | 21 | 0 | 21 | 99.6 (98.8–99.9) | 0.93 (0.85–1.0) |
| − | 3 | 681 | 684 | |||
| CSCT | + | 22 | 2 | 24 | 99.4 (98.6–99.8) | 0.91 (0.83–1.0) |
| − | 2 | 680 | 682 | |||
| PC L-Pap | + | 22 | 2 | 24 | 99.4 (98.5–99.8) | 0.91 (0.83–1.0) |
| − | 2 | 669 | 671 | |||
| SP L-Pap | + | 16 | 0 | 16 | 99.3 (98.1–99.7) | 0.89 (0.77–1.0) |
| − | 4 | 518 | 522 | |||
S-VSCT, self-collected vaginal specimen collection and transportation sample; P-VSCT, physician-collected vaginal specimen collection and transportation sample; CSCT, cervical specimen collection and transportation sample; PC, PerservCyt sample; SP, SurePath sample. +, positive; −, negative.
TABLE 2.
Overall Agreement of S-VSCT samples with P-VSCT, CSCT, PC, and SP samples for T. vaginalisa
| Sample | Result | No. of S-VSCT samples |
% overall agreement (95% CI) | Kappa (95% CI) | ||
|---|---|---|---|---|---|---|
| + | − | Total | ||||
| P-VSCT | + | 19 | 1 | 20 | 99.6 (98.8–99.9) | 0.93 (0.85–1.0) |
| − | 0 | 676 | 676 | |||
| CSCT | + | 17 | 0 | 17 | 99.6 (99.2–100) | 0.97 (0.92–1.0) |
| − | 2 | 675 | 677 | |||
| PC L-Pap | + | 16 | 0 | 16 | 99.6 (98.5–99.8) | 0.91 (0.81–1.0) |
| − | 3 | 668 | 671 | |||
| SP L-Pap | + | 11 | 0 | 11 | 98.9 (97.6–99.5) | 0.78 (0.61–0.96) |
| − | 6 | 517 | 523 | |||
S-VSCT, self-collected vaginal specimen collection and transportation sample; P-VSCT, physician-collected vaginal specimen collection and transportation sample; CSCT, cervical specimen collection and transportation sample; PC, PerservCyt sample; SP, SurePath sample. +, positive; −, negative.
Although there was good agreement in the current study between S-VSCT and SP L-Pap samples (99.3% for C. trachomatis and 98.9% for T. vaginalis), the kappa values were 0.89 for C. trachomatis and 0.78 for T. vaginalis. Fewer C. trachomatis and T. vaginalis infections were detected in SP L-Pap samples than in PC L-Pap samples, as reported in 3 previous studies (15–17). Differences between PC and SP L-Pap sensitivity values may be due to order of collection in the study or due to different ingredients in the two L-Pap transportation fluids. SP L-Pap contains chemicals which can induce nucleic acid cross-linking and effect RNA integrity, causing false-negative results with time (18, 19). This phenomenon can be reversed to some extent by proteinase K and heat treatment of the SP L-Pap samples (20), although this was not attempted in this study.
In conclusion, the high level of acceptability of the new SCT collection kit for vaginal self-sampling, including ease of collection and comfort, plus the strong agreement of self-collected vaginal sampling with physician-collected cervical and vaginal samples, provides good evidence for its use in testing for C. trachomatis and T. vaginalis from these sample types.
ACKNOWLEDGMENTS
This study was funded by Hologic/GenProbe Inc.
J. Reid and C. Hill are paid employees of Hologic GenProbe.
Footnotes
Published ahead of print 4 December 2013
REFERENCES
- 1.Kapala J, Jang D, Patel J, Biers K, Smieja M, Chernesky M. 2007. Pap cytology and the presence of high-risk human papillomavirus in SurePathTM liquid preservative and Digene cervical sampler specimens. J. Virol. Methods 142:223–225. 10.1016/j.jviromet.2007.01.028 [DOI] [PubMed] [Google Scholar]
- 2.Chernesky M, Huang S, Jang D, Erickson B, Saliture J, Engel H, Gilchrist J, Neuscheler P, Mak WB, Abravaya K. 2012. Performance of a new HPV Cervi-Collect collection and transportation kit. J. Oncol. 2012:503432. 10.1155/2012/503432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Newman SB, Nelson MB, Gaydos CA, Friedman HB. 2003. Female prisoners' preferences of collection methods for testing Chlamydia trachomatis and Neisseria gonorrhoeae infection. Sex. Transm. Dis. 30:306–309. 10.1097/00007435-200304000-00006 [DOI] [PubMed] [Google Scholar]
- 4.Richardson E, Sellors JW, Mackinnon S, Woodcox V, Howard M, Jang D, Karwalajtys T, Chernesky MA. 2003. Prevalence of Chlamydia trachomatis infections and specimen collection preference among women, using self-collected vaginal swabs in community settings. Sex. Transm. Dis. 30:880–885. 10.1097/01.OLQ.0000091142.68884.2A [DOI] [PubMed] [Google Scholar]
- 5.Dzuba IG, Díaz EY, Allen B, Leonard YF, Lazcano Ponce EC, Shah KV, Bishai D, Lorincz A, Ferris D, Turnbull B, Hernández Avila M, Salmerón J. 2002. The acceptability of self-collected samples for HPV testing vs. the Pap test as alternatives in cervical cancer screening. J. Womens Health Gend. Based Med. 11:265–275. 10.1089/152460902753668466 [DOI] [PubMed] [Google Scholar]
- 6.Chernesky MA, Hook EW, III, Martin DH, Lane J, Johnson R, Jordan JA, Fuller D, Willis DE, Fine PM, Janda WM, Schachter J. 2005. Women find it easy and prefer to collect their own vaginal swabs to diagnose Chlamydia trachomatis and Neisseria gonorrhoeae infections. Sex. Transm. Dis. 32:729–733. 10.1097/01.olq.0000190057.61633.8d [DOI] [PubMed] [Google Scholar]
- 7.Tisci S, Shen YH, Fife D, Huang J, Goycoolea J, Ma CP, Belinson J, Huang RD, Qiao YL. 2003. Patient acceptance of self-sampling for human papillomavirus in rural China. J. Low. Genit. Tract. Dis. 7:107–116. 10.1097/00128360-200304000-00007 [DOI] [PubMed] [Google Scholar]
- 8.Anhang R, Nelson JA, Telerant R, Chiasson MA, Wright TC., Jr 2005. Acceptability of self-collection of specimens for HPV DNA testing in an urban population. J. Womens Health 14:721–728. 10.1089/jwh.2005.14.721 [DOI] [PubMed] [Google Scholar]
- 9.Hsieh Y-H, Howell MR, Gaydos JC, McKee KT, Quinn TC, Gaydos CA. 2003. Preference among female Army recruits for us of self-administrated vaginal swabs for detection of Chlamydia trachomatis genital infections. Sex. Transm. Dis. 30:769–773. 10.1097/01.OLQ.0000079048.11771.46 [DOI] [PubMed] [Google Scholar]
- 10.Polaneczky M, Quigley C, Pollock L, Dulko D, Witkin SS. 1998. Use of self-collected vaginal specimens for detection of Chlamydia trachomatis infection. Obstet. Gynecol. 91:375–378. 10.1016/S0029-7844(97)00674-1 [DOI] [PubMed] [Google Scholar]
- 11.Forrest S, McCaffery K, Waller J, Desai M, Szarewski A, Cadman L, Wardle J. 2004. Attitudes to self-sampling for HPV among Indian, Pakistani, African-Caribbean and white British women in Manchester, UK. J. Med. Screen. 11:85–88. 10.1258/096914104774061065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kahn JA, Bernstein DI, Rosenthal SL, Huang B, Kollar LM, Colyer JL, Tissot AM, Hillard PA, Witte D, Groen P, Slap GB. 2005. Acceptability of human papillomavirus self-testing in female adolescents. Sex. Transm. Infect. 81:408–414. 10.1136/sti.2004.012047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Serlin M, Shafer MA, Tebb K, Gyamfi AA, Moncada J, Schachter J, Wibbelsman C. 2002. What sexually transmitted disease screening method does the adolescent prefer? Adolescents' attitudes toward first-void urine, self-collected vaginal swab, and pelvic examination. Arch. Pediatr. Adolesc. Med. 156:588–591. 10.1001/archpedi.156.6.588 [DOI] [PubMed] [Google Scholar]
- 14.Ginocchio CC, Chapin K, Smith JS, Aslanzadeh J, Snook J, Hill CS, Gaydos CA. 2012. Prevalence of Trichomonas vaginalis coinfection with Chlamydia trachomatis and Neisseria gonorrhoeae in the United States as determined by APTIMA Trichomonas vaginalis nucleic acid amplification assay. J. Clin. Microbiol. 50:2601–2608. 10.1128/JCM.00748-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chernesky M, Freund GG, Hook E, III, Leone P, D'Ascoli P, Martens M. 2007. Detection of Chlamydia trachomatis and Neisseria gonorrhoeae infections in North American women testing SurePath liquid-based Pap specimens. J. Clin. Microbiol. 45:2434–2438. 10.1128/JCM.00013-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chernesky M, Jang D, Smieja M, Portillo E, Ewert R, Pritchard C, MacEachern D, Doucette C, MacDonald A, Kapala J, Sumner J, Hill C. 2009. Validation of the APTIMA Combo 2 assay for the detection of Chlamydia trachomatis and Neisseria gonorrhoeae in SurePath liquid-based Pap test samples taken with different collection devices. Sex. Transm. Dis. 36:581–583. 10.1097/OLQ.0b013e3181a4c3fc [DOI] [PubMed] [Google Scholar]
- 17.Khader SN, Schlesinger K, Grossman J, Henry RI, Suhrland M, Fox AS. 2010. APTIMA assay on SurePath liquid-based cervical samples compared to endocervical swab samples facilitated by a real time database. Cytojournal 7:11. 10.4103/1742-6413.65057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horvath CA, Boulet G, Sahebali S, Depuydt C, Vermeulen T, Vanden Broeck D, Vereecken A, Bogers J. 2008. Effects of fixation on RNA integrity in a liquid-based cervical cytology setting. J. Clin. Pathol. 61:132–137 [DOI] [PubMed] [Google Scholar]
- 19.Powell N, Smith K, Fiander A. 2006. Recovery of human papillomavirus nucleic acids from liquid-based cytology media. J. Virol. Methods 137:58–62. 10.1016/j.jviromet.2006.05.033 [DOI] [PubMed] [Google Scholar]
- 20.Chernesky M, Gilchrist J, Jang D, Toor R, Schroder A, Dockter J. 2012. Effect of proteinase K treatment of SurePath L-Pap samples on detection of HPV mRNA. Eurogin International Multidisciplinary Congress Meeting, 8–11 July 2012, Prague, Czech Republic [Google Scholar]