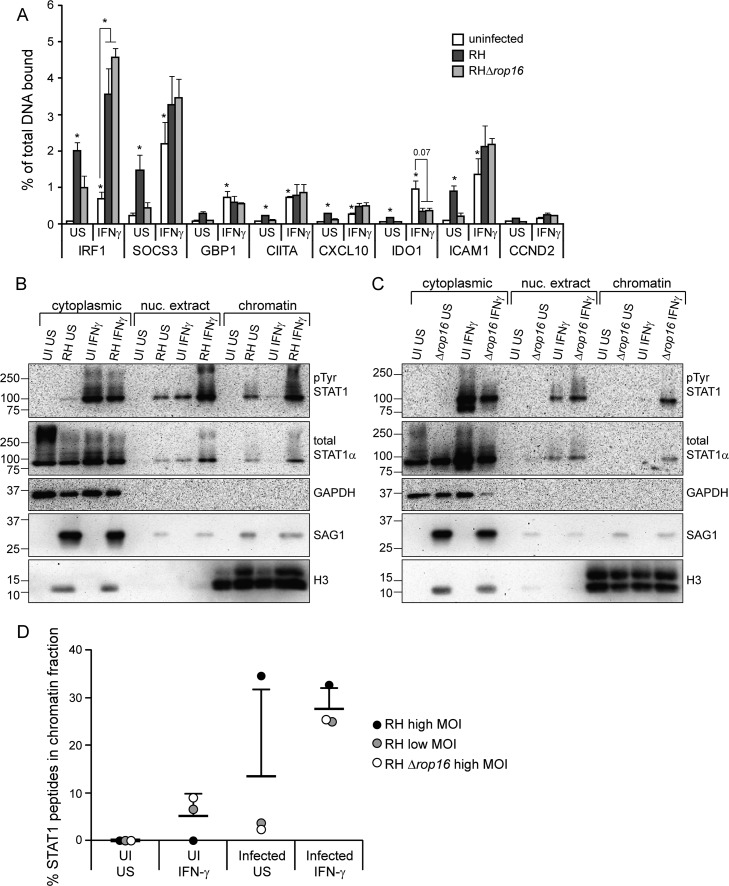
FIG 4.
IFN-γ-induced STAT1 DNA association is increased upon infection with Toxoplasma. HFFs were infected with RH or RHΔrop16 parasites, or left uninfected (UI), for 4 to 5 h. Cells were stimulated with 100 U of human IFN-γ/ml for the last hour of infection or left unstimulated (US). (A) Samples were fixed with 1% formaldehyde and collected for chromatin immunoprecipitation. qPCR of STAT1-binding regions of the promoters of IFN-γ-responsive genes was performed on both the immunoprecipitated STAT1-bound DNA and total input DNA. The percentage of the total DNA bound by STAT1 was calculated. A promoter region where STAT1 is not known to bind (CCND2) was also included as a negative control. The averages and SEM of three experiments are shown. The average MOI in the three experiments was 8. Asterisks (*) indicate P < 0.05 versus the uninfected, unstimulated sample, or the P value is indicated above the bars. (B to D) Samples were fractionated in cytoplasmic, nuclear extract, and chromatin fractions. Then, 1/4 to 1/6 of each fraction was diluted in 2× reducing sample buffer and boiled, and the protein levels were analyzed by SDS-PAGE and Western blotting (B and C). GAPDH and H3 are markers for cytoplasmic and chromatin fractions, respectively. The H3 antibody cross-reacts with Toxoplasma H3 and is therefore also found in the cytoplasmic fraction. STAT1 was then immunoprecipitated from the remaining portion of all three fractions, and mass spectrometry was performed. From each sample, the percentage of the total STAT1 peptides that were found in the chromatin fraction was calculated (D). The data are from three experiments performed independently at different times: HFFs infected with RH at a plaque assay-calculated MOI of ∼8 (B and D), RH at a plaque assay-calculated MOI of ∼1.5 (D), and RHΔrop16 at a plaque assay-calculated MOI of ∼5 (C and D).