FIG 2.
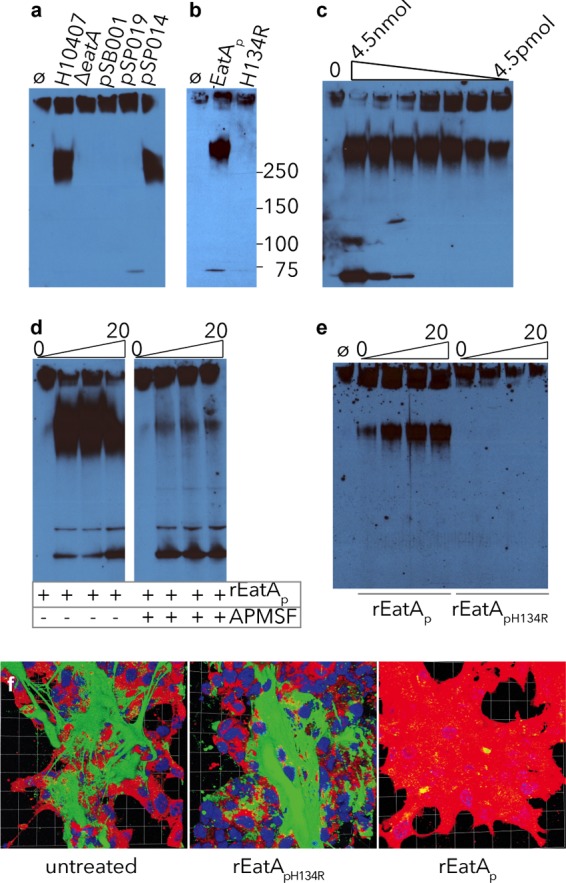
Mucin degradation by EatA requires serine protease activity. Using concentrated culture supernatant from the eatA mutant, we observed no MUC2 degradation compared to that from WT ETEC H10407. (a) Complementation of the eatA mutant with WT EatA (pSP014), but not H134R mutant EatA (pSP019) or vector control (pSB001), restored activity. (b) H134R mutant EatA (pSP019) purified from E. coli LMG194ΔfliC was unable to degrade MUC2 compared to WT EatA (pSP014). (c) rEatAp degrades purified MUC2 in a dose-dependent fashion. Untreated MUC2 is shown in the leftmost lane (lane 0) followed by serial dilutions of rEatAp passenger domain starting at 4.5 nmol/ml of protein. (d) Protease activity of rEatAp was inhibited by APMSF. (e) MUC2 is degraded in a time-dependent manner (samples obtained at 0, 5, 10, and 20 minutes) by the purified passenger domain of recombinant wild-type EatA (rEatAp) but not by the purified active-site mutant protein (rEatApH134R). (f) EatA degrades cell-associated MUC2. Abundant MUC2 (green) on the surfaces of untreated LS174T cells and monolayers treated with recombinant H134R mutant EatA passenger protein (rEatApH134R) compared to cells treated with recombinant wild-type EatA passenger domain (rEatAp). The images shown in panel f are Volocity-generated 3D projections from z-stacks.
