Abstract
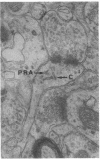
Animals placed in complex environments develop greater numbers of visual cortex synapses per neuron than animals housed in standard cages. Increased numbers of synapses could theoretically arise from (i) active formation of new synapses, or (ii) selective stabilization of constitutively produced synapses. The postsynaptic location of polyribosomal aggregates appears to be an indicator of newly forming synapses. In developmental synaptogenesis and adult reactive (to injury) synaptogenesis, polyribosomes are more frequently found at spine synapses and are more likely to appear in the spine head and stem. In the visual cortex of rats from complex environments, there was a greater frequency of spine synapses associated with polyribosomes, relative to rats from individual or group cages. Furthermore, a greater percentage of these spines had polyribosomes in the head and stem region. This suggests that synapses in this region may be actively induced by neural activity arising from the complex environment experience.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhide P. G., Bedi K. S. The effects of a lengthy period of environmental diversity on well-fed and previously undernourished rats. II. Synapse-to-neuron ratios. J Comp Neurol. 1984 Aug 1;227(2):305–310. doi: 10.1002/cne.902270213. [DOI] [PubMed] [Google Scholar]
- Chang F. L., Greenough W. T. Lateralized effects of monocular training on dendritic branching in adult split-brain rats. Brain Res. 1982 Jan 28;232(2):283–292. doi: 10.1016/0006-8993(82)90274-8. [DOI] [PubMed] [Google Scholar]
- Chang F. L., Greenough W. T. Transient and enduring morphological correlates of synaptic activity and efficacy change in the rat hippocampal slice. Brain Res. 1984 Aug 20;309(1):35–46. doi: 10.1016/0006-8993(84)91008-4. [DOI] [PubMed] [Google Scholar]
- Changeux J. P., Danchin A. Selective stabilisation of developing synapses as a mechanism for the specification of neuronal networks. Nature. 1976 Dec 23;264(5588):705–712. doi: 10.1038/264705a0. [DOI] [PubMed] [Google Scholar]
- Changeux J. P., Devillers-Thiéry A., Chemouilli P. Acetylcholine receptor: an allosteric protein. Science. 1984 Sep 21;225(4668):1335–1345. doi: 10.1126/science.6382611. [DOI] [PubMed] [Google Scholar]
- Coleman P. D., Riesen A. H. Evironmental effects on cortical dendritic fields. I. Rearing in the dark. J Anat. 1968 Mar;102(Pt 3):363–374. [PMC free article] [PubMed] [Google Scholar]
- Cotman C. W., Nieto-Sampedro M. Brain function, synapse renewal, and plasticity. Annu Rev Psychol. 1982;33:371–401. doi: 10.1146/annurev.ps.33.020182.002103. [DOI] [PubMed] [Google Scholar]
- Cragg B. G. Changes in visual cortex on first exposure of rats to light. Effect on synaptic dimensions. Nature. 1967 Jul 15;215(5098):251–253. doi: 10.1038/215251a0. [DOI] [PubMed] [Google Scholar]
- Cragg B. G. The development of synapses in kitten visual cortex during visual deprivation. Exp Neurol. 1975 Mar;46(3):445–451. doi: 10.1016/0014-4886(75)90118-1. [DOI] [PubMed] [Google Scholar]
- Dunn A. J. Neurochemistry of learning and memory: an evaluation of recent data. Annu Rev Psychol. 1980;31:343–390. doi: 10.1146/annurev.ps.31.020180.002015. [DOI] [PubMed] [Google Scholar]
- Fambrough D. M. Control of acetylcholine receptors in skeletal muscle. Physiol Rev. 1979 Jan;59(1):165–227. doi: 10.1152/physrev.1979.59.1.165. [DOI] [PubMed] [Google Scholar]
- Fass B., Steward O. Increases in protein-precursor incorporation in the denervated neuropil of the dentate gyrus during reinnervation. Neuroscience. 1983 Jul;9(3):653–664. doi: 10.1016/0306-4522(83)90181-1. [DOI] [PubMed] [Google Scholar]
- Fifková E. The effect of monocular deprivation on the synaptic contacts of the visual cortex. J Neurobiol. 1969;1(3):285–294. doi: 10.1002/neu.480010304. [DOI] [PubMed] [Google Scholar]
- Fifková E. The effect of unilateral deprivation on visual centers in rats. J Comp Neurol. 1970 Dec;140(4):431–438. doi: 10.1002/cne.901400404. [DOI] [PubMed] [Google Scholar]
- Freire M. Effects of dark rearing on dendritic spines in layer IV of the mouse visual cortex. A quantitative electron microscopical study. J Anat. 1978 May;126(Pt 1):193–201. [PMC free article] [PubMed] [Google Scholar]
- GRAY E. G. Axo-somatic and axo-dendritic synapses of the cerebral cortex: an electron microscope study. J Anat. 1959 Oct;93:420–433. [PMC free article] [PubMed] [Google Scholar]
- GRAY E. G., GUILLERY R. W. A NOTE ON THE DENDRITIC SPINE APPARATUS. J Anat. 1963 Jul;97:389–392. [PMC free article] [PubMed] [Google Scholar]
- Globus A., Rosenzweig M. R., Bennett E. L., Diamond M. C. Effects of differential experience on dendritic spine counts in rat cerebral cortex. J Comp Physiol Psychol. 1973 Feb;82(2):175–181. doi: 10.1037/h0033910. [DOI] [PubMed] [Google Scholar]
- Green E. J., Greenough W. T., Schlumpf B. E. Effects of complex or isolated environments on cortical dendrites of middle-aged rats. Brain Res. 1983 Apr 4;264(2):233–240. doi: 10.1016/0006-8993(83)90821-1. [DOI] [PubMed] [Google Scholar]
- Greenough W. T., Juraska J. M., Volkmar F. R. Maze training effects on dendritic branching in occipital cortex of adult rats. Behav Neural Biol. 1979 Jul;26(3):287–297. doi: 10.1016/s0163-1047(79)91278-0. [DOI] [PubMed] [Google Scholar]
- Greenough W. T., Volkmar F. R. Pattern of dendritic branching in occipital cortex of rats reared in complex environments. Exp Neurol. 1973 Aug;40(2):491–504. doi: 10.1016/0014-4886(73)90090-3. [DOI] [PubMed] [Google Scholar]
- Greenough W. T., West R. W., DeVoogd T. J. Subsynaptic plate perforations: changes with age and experience in the rat. Science. 1978 Dec 8;202(4372):1096–1098. doi: 10.1126/science.715459. [DOI] [PubMed] [Google Scholar]
- Juraska J. M., Greenough W. T., Elliott C., Mack K. J., Berkowitz R. Plasticity in adult rat visual cortex: an examination of several cell populations after differential rearing. Behav Neural Biol. 1980 Jun;29(2):157–167. doi: 10.1016/s0163-1047(80)90482-3. [DOI] [PubMed] [Google Scholar]
- Juraska J. M. Sex differences in dendritic response to differential experience in the rat visual cortex. Brain Res. 1984 Mar 12;295(1):27–34. doi: 10.1016/0006-8993(84)90812-6. [DOI] [PubMed] [Google Scholar]
- Levitan I. B., Mushynski W. E., Ramirez G. Effects of environmental complexity on amino acid incorporation into rat cortex and hippocampus in vivo. J Neurochem. 1972 Nov;19(11):2621–2630. doi: 10.1111/j.1471-4159.1972.tb01321.x. [DOI] [PubMed] [Google Scholar]
- Purves D., Lichtman J. W. Elimination of synapses in the developing nervous system. Science. 1980 Oct 10;210(4466):153–157. doi: 10.1126/science.7414326. [DOI] [PubMed] [Google Scholar]
- Rothblat L. A., Schwartz M. L. The effect of monocular deprivation on dendritic spines in visual cortex of young and adult albino rats: evidence for a sensitive period. Brain Res. 1979 Jan 26;161(1):156–161. doi: 10.1016/0006-8993(79)90203-8. [DOI] [PubMed] [Google Scholar]
- Steward O. Alterations in polyribosomes associated with dendritic spines during the reinnervation of the dentate gyrus of the adult rat. J Neurosci. 1983 Jan;3(1):177–188. doi: 10.1523/JNEUROSCI.03-01-00177.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward O., Levy W. B. Preferential localization of polyribosomes under the base of dendritic spines in granule cells of the dentate gyrus. J Neurosci. 1982 Mar;2(3):284–291. doi: 10.1523/JNEUROSCI.02-03-00284.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward O. Polyribosomes at the base of dendritic spines of central nervous system neurons--their possible role in synapse construction and modification. Cold Spring Harb Symp Quant Biol. 1983;48(Pt 2):745–759. doi: 10.1101/sqb.1983.048.01.077. [DOI] [PubMed] [Google Scholar]
- Turner A. M., Greenough W. T. Differential rearing effects on rat visual cortex synapses. I. Synaptic and neuronal density and synapses per neuron. Brain Res. 1985 Mar 11;329(1-2):195–203. doi: 10.1016/0006-8993(85)90525-6. [DOI] [PubMed] [Google Scholar]
- Valverde F. Rate and extent of recovery from dark rearing in the visual cortex of the mouse. Brain Res. 1971 Oct 8;33(1):1–11. doi: 10.1016/0006-8993(71)90302-7. [DOI] [PubMed] [Google Scholar]
- Volkmar F. R., Greenough W. T. Rearing complexity affects branching of dendrites in the visual cortex of the rat. Science. 1972 Jun 30;176(4042):1445–1447. doi: 10.1126/science.176.4042.1445. [DOI] [PubMed] [Google Scholar]
- Wesa J. M., Chang F. L., Greenough W. T., West R. W. Synaptic contact curvature: effects of differential rearing on rat occipital cortex. Brain Res. 1982 Jun;256(2):253–257. doi: 10.1016/0165-3806(82)90049-9. [DOI] [PubMed] [Google Scholar]
- West R. W., Greenough W. T. Effect of environmental complexity on cortical synapses of rats: preliminary results. Behav Biol. 1972 Apr;7(2):279–284. doi: 10.1016/s0091-6773(72)80207-4. [DOI] [PubMed] [Google Scholar]