Abstract
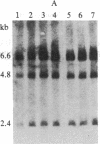
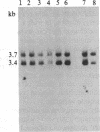
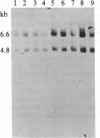
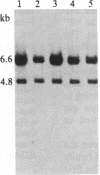
Achondroplasia is an autosomal dominant disorder that involves defective endochondral bone formation. Type II collagen is the predominant collagen of cartilage. We found a HindIII polymorphic site in the normal Caucasian population by using the type II procollagen gene probe pgHCol(II)A. The presence of this site yields a 7.0-kilobase (kb) band; its absence yields a 14.0-kb band. We found a significant deviation in genotype distribution and allele frequencies in a population of unrelated individuals with sporadic achondroplasia, compared with the normal control population. The HindIII genotype frequencies in 32 individuals with achondroplasia are 0.41 for the 7/7 genotype (controls, 0.08), 0.34 for the 7/14 genotype (controls, 0.54), and 0.25 for the 14/14 genotype (controls, 0.37). The apparent equilibrium excess of the "7" allele in individuals with achondroplasia may reflect either a predisposition for the mutation that causes achondroplasia or it could be the result of the achondroplasia-causing mutation. In either case, these findings suggest an association of the type II procollagen gene with achondroplasia.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldridge J., Kunkel L., Bruns G., Tantravahi U., Lalande M., Brewster T., Moreau E., Wilson M., Bromley W., Roderick T. A strategy to reveal high-frequency RFLPs along the human X chromosome. Am J Hum Genet. 1984 May;36(3):546–564. [PMC free article] [PubMed] [Google Scholar]
- Bornstein P., Sage H. Structurally distinct collagen types. Annu Rev Biochem. 1980;49:957–1003. doi: 10.1146/annurev.bi.49.070180.004521. [DOI] [PubMed] [Google Scholar]
- Davis M. M., Kim S. K., Hood L. Immunoglobulin class switching: developmentally regulated DNA rearrangements during differentiation. Cell. 1980 Nov;22(1 Pt 1):1–2. doi: 10.1016/0092-8674(80)90145-2. [DOI] [PubMed] [Google Scholar]
- Fietzek P. P., Kühn K. The primary structure of collagen. Int Rev Connect Tissue Res. 1976;7:1–60. doi: 10.1016/b978-0-12-363707-9.50007-1. [DOI] [PubMed] [Google Scholar]
- Lam S. T., Stahl M. M., McMilin K. D., Stahl F. W. Rec-mediated recombinational hot spot activity in bacteriophage lambda. II. A mutation which causes hot spot activity. Genetics. 1974 Jul;77(3):425–433. doi: 10.1093/genetics/77.3.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer L. O., Jr, Baumann P. A., Gorlin R. J. Achondroplasia. Am J Roentgenol Radium Ther Nucl Med. 1967 May;100(1):12–26. doi: 10.2214/ajr.100.1.12. [DOI] [PubMed] [Google Scholar]
- Malone R. E., Chattoraj D. K., Faulds D. H., Stahl M. M., Stahl F. W. Hotspots for generalized recombination in the Escherichia coli chromosome. J Mol Biol. 1978 Jun 5;121(4):473–491. doi: 10.1016/0022-2836(78)90395-9. [DOI] [PubMed] [Google Scholar]
- Oberklaid F., Danks D. M., Jensen F., Stace L., Rosshandler S. Achondroplasia and hypochondroplasia. Comments on frequency, mutation rate, and radiological features in skull and spine. J Med Genet. 1979 Apr;16(2):140–146. doi: 10.1136/jmg.16.2.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opitz J. M. "Unstable premutation" in achondroplasia: penetrance vs phenotrance. Am J Med Genet. 1984 Oct;19(2):251–254. doi: 10.1002/ajmg.1320190207. [DOI] [PubMed] [Google Scholar]
- Rimoin D. L., Silberberg R., Hollister D. W. Chondro-osseous pathology in the chondrodystrophies. Clin Orthop Relat Res. 1976 Jan-Feb;(114):137–152. [PubMed] [Google Scholar]
- Sakano H., Maki R., Kurosawa Y., Roeder W., Tonegawa S. Two types of somatic recombination are necessary for the generation of complete immunoglobulin heavy-chain genes. Nature. 1980 Aug 14;286(5774):676–683. doi: 10.1038/286676a0. [DOI] [PubMed] [Google Scholar]
- Sandell L. J., Prentice H. L., Kravis D., Upholt W. B. Structure and sequence of the chicken type II procollagen gene. Characterization of the region encoding the carboxyl-terminal telopeptide and propeptide. J Biol Chem. 1984 Jun 25;259(12):7826–7834. [PubMed] [Google Scholar]
- Sillence D. O., Horton W. A., Rimoin D. L. Morphologic studies in the skeletal dysplasias. Am J Pathol. 1979 Sep;96(3):813–870. [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Strom C. M., Eddy R. L., Shows T. B. Localization of human type II procollagen gene (COL2A1) to chromosome 12. Somat Cell Mol Genet. 1984 Nov;10(6):651–655. doi: 10.1007/BF01535232. [DOI] [PubMed] [Google Scholar]
- Strom C. M., Upholt W. B. Isolation and characterization of genomic clones corresponding to the human type II procollagen gene. Nucleic Acids Res. 1984 Jan 25;12(2):1025–1038. doi: 10.1093/nar/12.2.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynne-Davies R., Walsh W. K., Gormley J. Achondroplasia and hypochondroplasia. Clinical variation and spinal stenosis. J Bone Joint Surg Br. 1981;63B(4):508–515. doi: 10.1302/0301-620X.63B4.7298674. [DOI] [PubMed] [Google Scholar]
- von der Mark K. Immunological studies on collagen type transition in chondrogenesis. Curr Top Dev Biol. 1980;14(Pt 2):199–225. doi: 10.1016/s0070-2153(08)60195-7. [DOI] [PubMed] [Google Scholar]