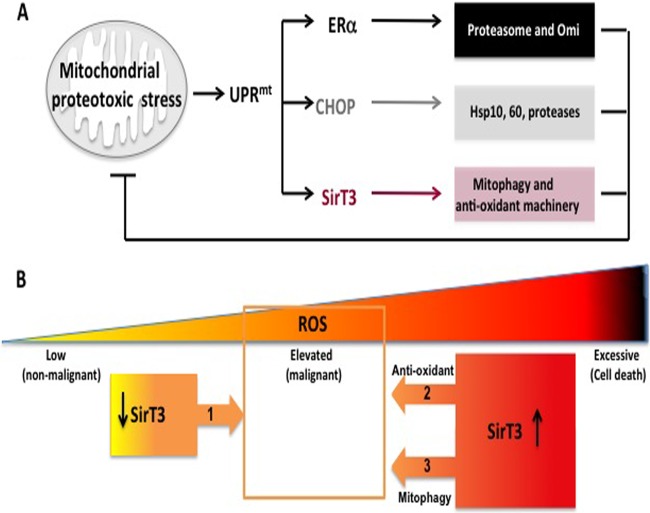
FIG 7.
(A) Diagram of the critical effectors of the UPRmt. Mitochondrial proteotoxic stress induces the UPRmt, which involves activation of CHOP, ERα, and SirT3. ERα, which is activated only in estrogen receptor-positive breast cancer cells, promotes the activity of the proteasome and of the protease Omi to limit the accumulation of misfolded proteins in the mitochondria. CHOP activates the heat shock proteins Hsp10 and Hsp60 as well as the protease LonP, which collectively also prevent the accumulation of misfolded proteins in the mitochondria. SirT3, described in the current study, confers additional but essential cytoprotective effects by activating antioxidant defense and mitophagy. SirT3 is critical for cellular viability under conditions of mitochondrial proteotoxic stress. Collectively, the combined outcome of the UPRmt is the reduction of proteotoxic stress and the maintenance of the integrity of the organelle. (B) SirT3 plays a dual role in breast cancer. On the one hand, a reduction in SirT3 levels is necessary to allow an increase in ROS levels and assist in the metabolic reprogramming of the mitochondria during transformation (arrow 1). On the other hand, by inducing the antioxidant machinery (arrow 2), SirT3 is required to repress ROS levels within a window (indicated by the orange square) that is compatible with the maintenance of cellular viability. In addition, by inducing the transcription of mitophagy genes (arrow 3), SirT3 primes the cells for the elimination of severely damaged mitochondria. However, the selective recruitment of the mitophagy machinery to these damaged organelles requires an additional step, which remains unknown. As these severely damaged organelles have excessive ROS, their elimination also contributes to reducing ROS within a window that is compatible with cellular viability (arrow 3). In the absence of SirT3, ROS increase to excessive levels, leading to cell death.