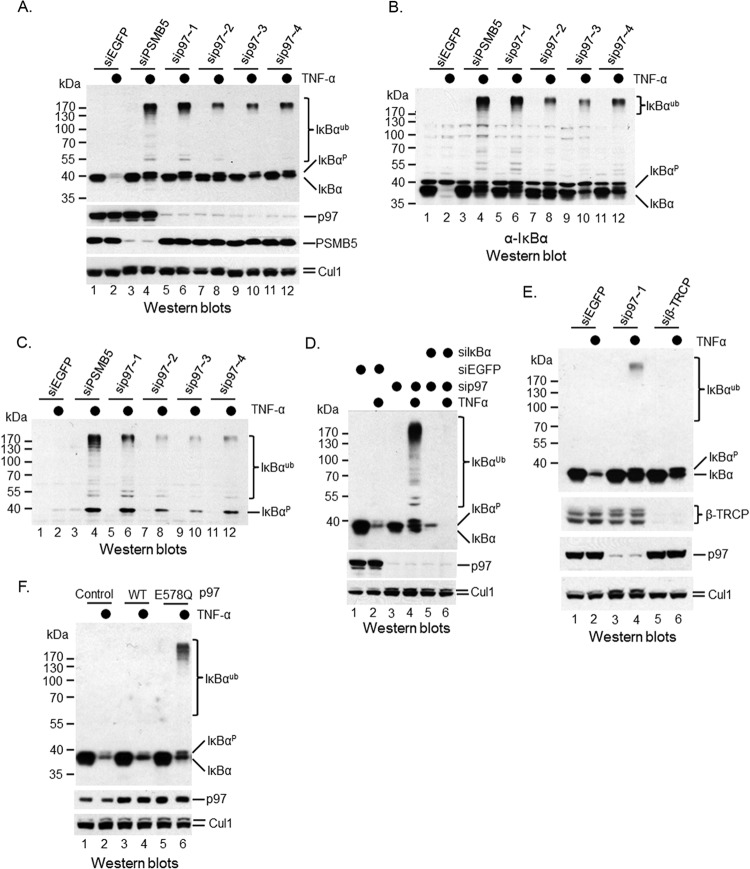
FIG 1.
p97 is required for TNF-α-induced IκBα degradation. HeLa cells were subjected to RNAi of PSMB5, p97, or β-TRCP as described in Materials and Methods. Western blotting was performed with anti-IκBα antibodies to show the dynamic change of IκBα under TNF-α treatments. The p97 antibody is from Cell Signaling. (A) Silencing of either PSMB5 or p97 blocked TNF-α-induced IκBα proteolysis. (B) The blot shown in panel A was stripped and then probed with a polyclonal anti-IκBα antibody. (C) Accumulation of phosphorylated IκBα upon either PSMB5 or p97 was depleted using siRNA. (D) Confirmation of both high- and low-molecular-mass IκBα proteins by cosilencing of p97 and IκBα. (E) β-TRCP depletion blocked TNF-α-induced IκBα ubiquitination. (F) The wild type and an ATPase-defective mutant, E578Q of p97, were delivered into HeLa cells using a lentiviral infection method. TNF-α was employed to trigger IκBα ubiquitination and degradation. A Western blotting method was employed to detect modified and unmodified IκBα.