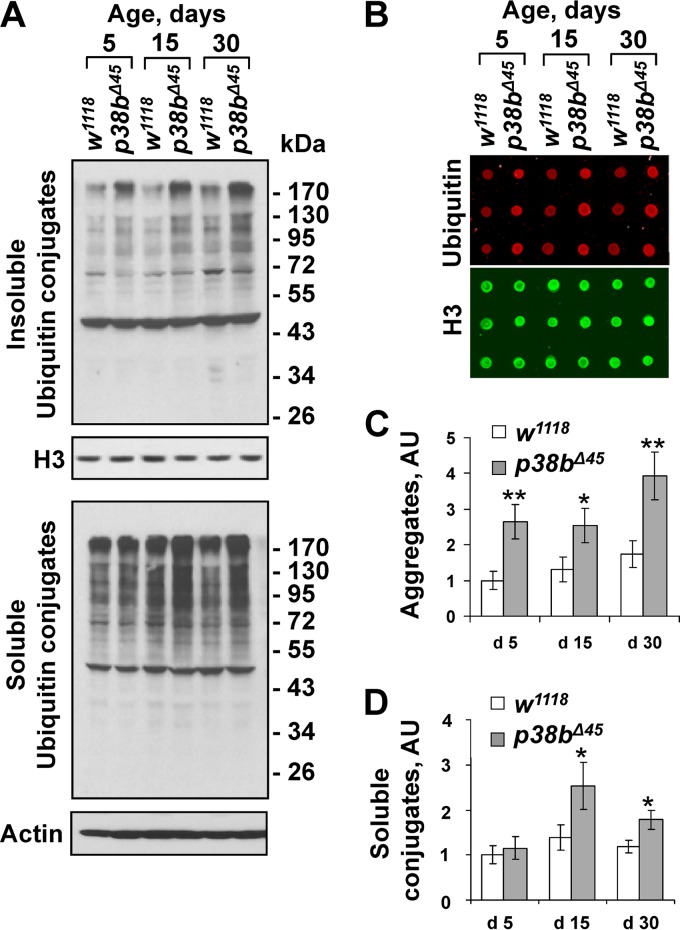
FIG 1.
p38MAPK regulates protein aggregate accumulation in aging thoracic muscle. (A) Enhanced accumulation of ubiquitylated protein aggregates in thoracic muscles of p38b-deficient flies. Triton X-100-insoluble (top) and Triton X-100-soluble (bottom) protein extracts were prepared from the thoraces of wild-type (w1118) and isogenic p38b-null (p38bΔ45) flies at 5, 15, and 30 days after eclosion. The levels of ubiquitylated conjugates were assayed using an antiubiquitin antibody. Actin and histone H3 were used as loading controls for soluble and insoluble extracts, respectively. (B) The levels of ubiquitylated aggregates were quantified by dot blot analyses using near-infrared fluorescent detection (see Materials and Methods). Dot blot assays were performed in triplicates. Red fluorescent signal (ubiquitin) was divided by green signal (histone H3) and normalized by the w1118 control at day 5. A representative dot blot with aggregate samples is shown. (C and D) Quantification of ubiquitin conjugates in aggregate and detergent-soluble fractions. Columns represent means ± the standard errors (SD; n = 3; NS, not significant; *, P < 0.05 [unpaired two-tailed Student t test]).