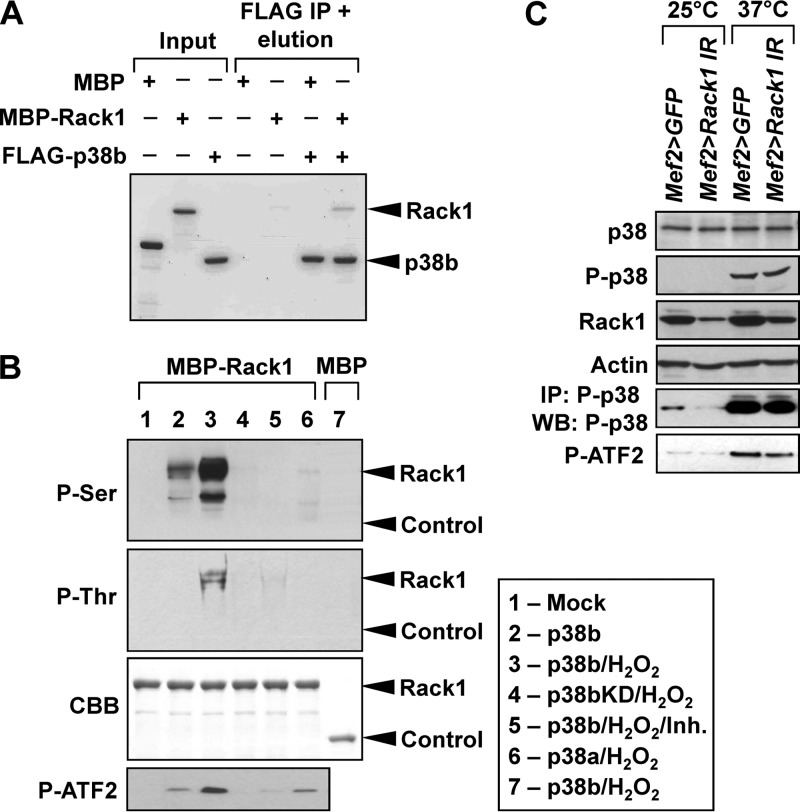
FIG 5.
Rack1 acts as a direct substrate of p38b without affecting its kinase activity. (A) Bacterially expressed MBP-Rack1 and FLAG-p38b exhibit binding in a pulldown assay, confirming their direct interaction. Proteins were visualized by Coomassie blue staining. Bands corresponding to p38b and Rack1 are marked. Band densitometry showed that ca. 5% of input Rack1 bound to p38b, a finding consistent with a transient kinase-substrate interaction. (B) S2 cells were transfected with the indicated FLAG-tagged p38MAPK constructs and stimulated by 10 mM H2O2 for 30 min. Kinases were then purified from S2 lysates by FLAG-immunoprecipitation and used in in vitro kinase assays with MBP-Rack1. MBP and ATF2 were used as controls. Inh, 10 μM SB203580. Following the kinase reactions, MBR-Rack1 was repurified by using amylose beads, and its phosphorylation was determined by Western blotting. The location of bands corresponding to MBP-Rack1 (Rack1) and MBP (Control) are indicated. The composition of the in vitro kinase reactions corresponding to gel lanes is shown in the box. (C) The p38b level and activity were examined in the muscle of unstressed (25°C) or heat-shocked (37°C, 30 min) Rack1 knockdown (Mef2>Rack1 IR) and control (Mef2>GFP) flies. Levels of total and phospho-p38b (T183, Y185) were determined by Western blotting and show no change in response to Rack1 depletion. Activated endogenous P-p38 was immunoprecipitated from thorax extracts and assayed in vitro using a recombinant substrate ATF2. The kinase activity of P-p38 appears unchanged in Mef2>Rack1 IR flies compared to controls.