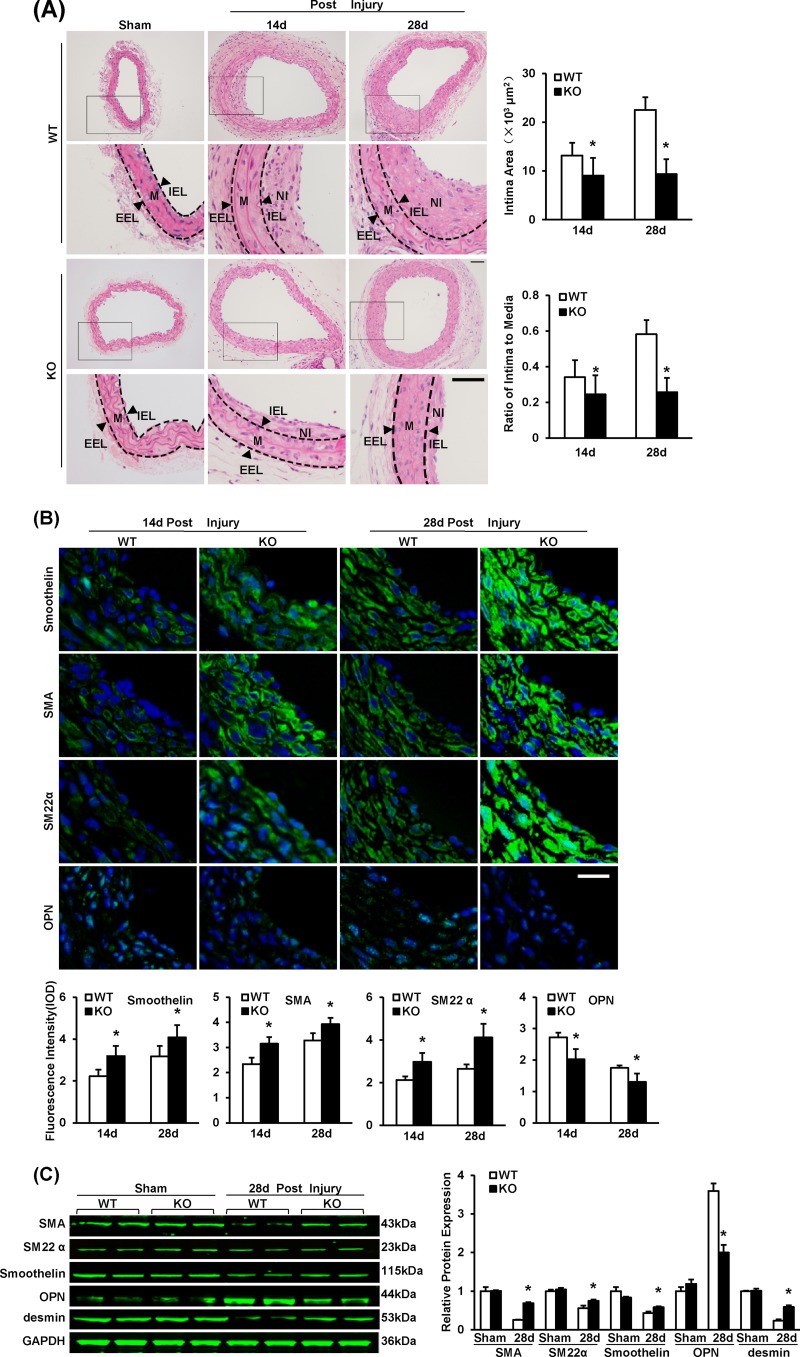
FIG 2.
IRF8 deficiency represses neointima formation and SMC phenotypic switching in response to artery injury. (A) HE-stained sections showing the structures of WT and IRF8−/− (KO) carotid arteries from mice that underwent a sham operation or wire injury surgery (at 14 and 28 days after surgery). Black-framed areas in the upper panels are magnified and shown in the lower panels. Arrowheads, internal elastic lamina (IEL) and external elastic lamina (EEL), highlighted in dotted lines. The internal elastic lamina indicates the intimal-medial boundary, while the external elastic lamina represents the medial-adventitial boundary. M, media; NI, neointima. Intimal areas and intima/media ratios were quantified (n = 9 to 12 per group at each time point). Bars, 50 μm. (B) Immunofluorescent staining of smoothelin, α-SMA, SM22α, and OPN (green in different rows) in WT and IRF8−/− arteries at 14 days and 28 days after injury. DAPI (blue) staining indicates nuclei. The optical density values of smoothelin, α-SMA, SM22α, and OPN fluorescence are also provided (n = 3 to 6 per group at each time point). Bar, 20 μm. (C) The levels of α-SMA, SM22α, smoothelin, OPN, and desmin protein in WT and IRF8−/− arteries were determined by Western blotting. Arteries harvested from the sham operation group and the groups at 28 days postinjury were used. The expression levels were normalized to the expression level of GAPDH and quantified. Blots are representative of three independent experiments. In panels A to C, all values are presented as means ± SDs, and statistical significance is indicated. *, P < 0.05 compared with the WT group.