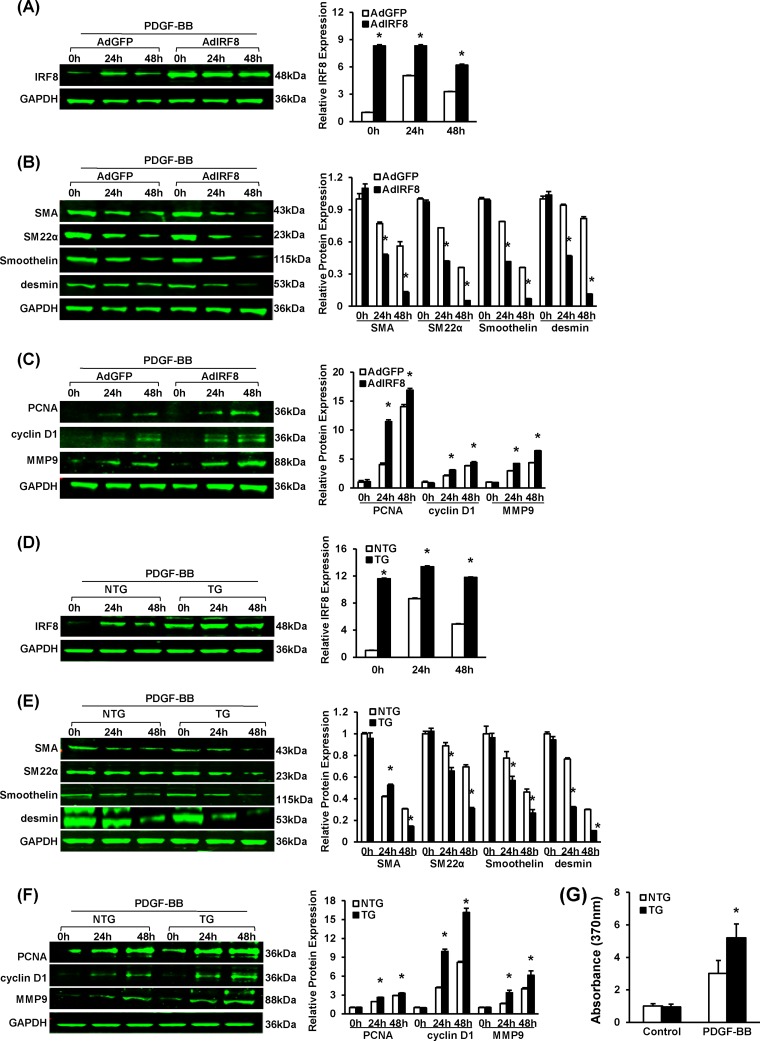
FIG 5.
SMC-specific IRF8 overexpression promotes SMC phenotypic switching upon PDGF-BB stimulation. (A) Western blotting validated the efficacy of adenovirus-mediated IRF8 overexpression in RASMCs upon PDGF-BB (20 ng/ml) stimulation. Cells were harvested before and at 24 and 48 h after PDGF-BB administration. (B) α-SMA, SM22α, smoothelin, and desmin protein levels in RASMCs infected with AdGFP and adenovirus expressing IRF8 (AdIRF8) were determined by Western blotting at different time points after PDGF-BB (20 ng/ml) administration. (C) PCNA, cyclin D1, and MMP9 protein levels in RASMCs infected with AdGFP and adenovirus expressing IRF8 were determined by Western blotting at different time points after PDGF-BB (20 ng/ml) administration. (D) Western blotting validated the IRF8 levels in nontransgenic and IRF8 TG mouse arterial smooth muscle cells in response to PDGF-BB (20 ng/ml) stimulation. Cells were harvested before and at 24 and 48 h after PDGF-BB administration. (E) α-SMA, SM22α, smoothelin, and desmin protein levels in nontransgenic and TG mouse SMCs were determined by Western blotting at different time points after PDGF-BB (20 ng/ml) administration. (F) PCNA, cyclin D1, and MMP9 protein levels in nontransgenic and TG mouse arteries were determined by Western blotting at different time points after PDGF-BB (20 ng/ml) administration. In panels A to F, the corresponding protein levels were normalized to the GAPDH level and quantified. These blots are representative of three blots obtained from three independent experiments. All of the values are presented as the means ± SDs, and the statistical significance is indicated. *, P < 0.05 compared with the AdGFP-treated or nontransgenic group. (G) BrdU incorporation was measured by determination of the absorption at 370 nm to assess nontransgenic and TG mouse VSMC proliferation upon PDGF-BB (20 ng/ml) stimulation. The values presented are the means ± SDs, and the statistical significance is indicated. *, P < 0.05 compared with the PDGF-BB-treated nontransgenic group (n = 4).