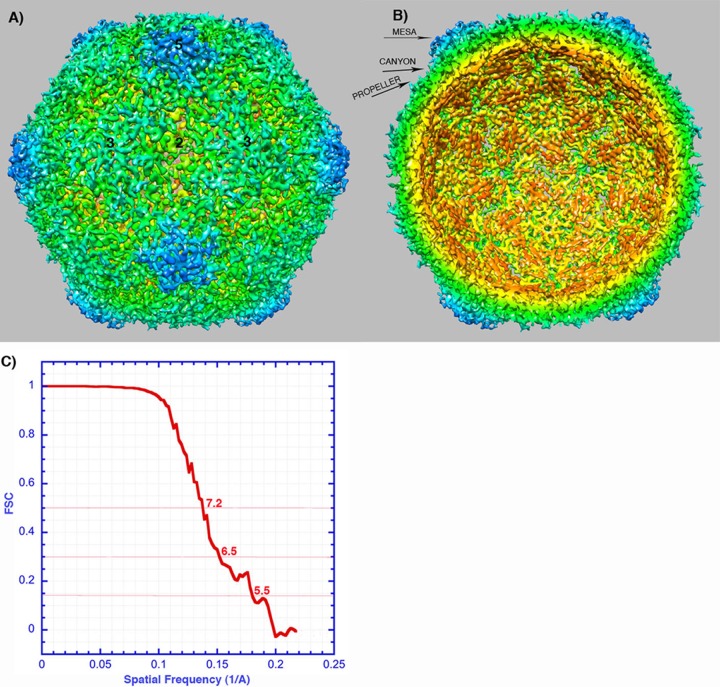
FIG 2.
Reconstruction of poliovirus 135S particles. (A and B) Surface rendering colored by radial position, showing the outer (A) and inner (B) surfaces. The map has been sharpened and displayed at high contour to emphasize higher-resolution details. In many places, individual polypeptide chains are resolved, which facilitates the rigid-body fitting of pseudoatomic models. Note the hole at the icosahedral 2-fold axis (center), where red-colored density is visible from the exterior. Labels indicate a mesa and a propeller tip, which are major projections from the outer surface. Separating the projections are canyons surrounding each 5-fold mesa and a saddle-shaped depression crossing the 2-fold axis. Symmetry axes are indicated by numbers. (C) A Fourier shell correlation (FSC) was calculated between randomly selected half-data sets. This curve suggests a resolution of ∼7 Å (at 0.5 correlation) and that there is meaningful information at ∼5.5 Å (the resolution at which FSC falls below 0.143).