FIG 5.
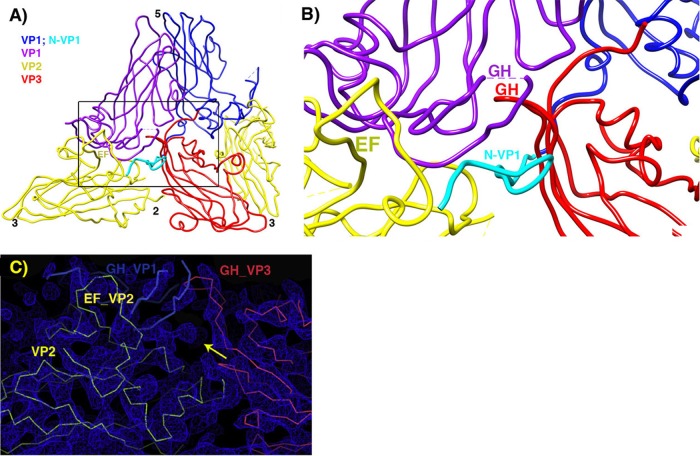
Pseudoatomic model in the vicinity of the quasi-3-fold hole. (A) Overview, to provide context. (B) Zoomed-in view. VP2 is yellow, and VP3 is red. Two symmetry copies of VP1 are purple and blue, with the rebuilt N-terminal extension of the blue copy shown in cyan. Following the example of the crystal structure of the 135S-like particles of coxsackie virus A16, a short stretch of the N terminus of VP1 (labeled in cyan) forms a third beta strand alongside the two-stranded beta sheet (labeled in red) that is created by the rearrangement of the GH loop of VP3. The distal end of the GH loop of VP1 (labeled in purple) has become reoriented radially and leans rightward to contact the 3-stranded beta structure. Hypothetically, these three polypeptide segments may serve as a nucleus for building additional ordered structure atop the VP2 beta barrel, in order for the uncoating process to proceed further, upon membrane interaction. (C) In the vicinity of the quasi-3-fold axis, a section of the reconstruction shows that strong linear electron density features are present that correspond to proximal portions of the GH loops of VP3 (red) and VP1 (blue), which have both adopted an extended conformation that is rearranged relative to mature virus. The externalized N-terminal extension of VP1 extends toward the viewer through the indicated density feature (yellow arrow).