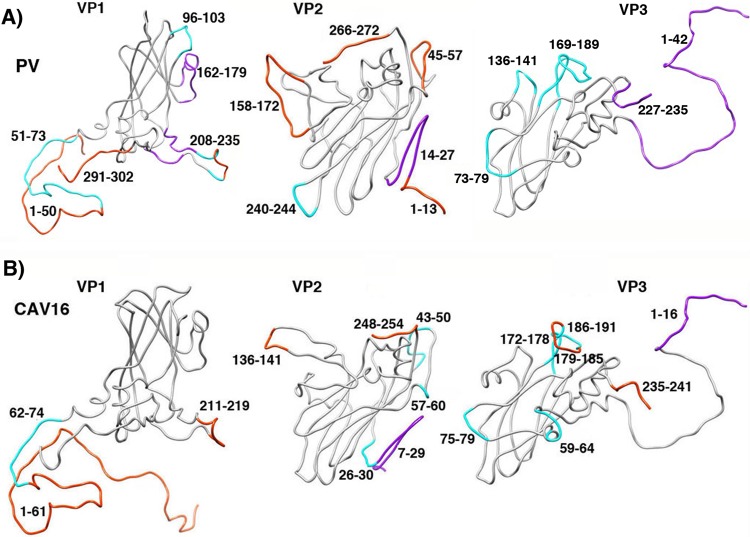
FIG 7.
The conformational changes seen in the expanded structures of other picornaviruses (such as the CAV16 135S particle [19]) (B) are less extensive than the ones seen in poliovirus 135S particles (A). Main chain traces from crystal structures of mature virions are shown for capsid proteins VP1, VP2, and VP3. The locations of polypeptide segments that become shifted, rearranged significantly, or disordered are colored magenta, cyan, and orange, respectively. The greater extent of changes in poliovirus 135S particles (A) might be the result of inherent lesser stability or simply greater heterogeneity in cryo-EM preparations, versus the preparations of expanded forms of other viruses that have been crystallized (15, 16, 19). In either case, our hypothesis is that much of the poliovirus population must be relatively further along the uncoating pathway. Panel A is Fig. 3B, repeated here for clarity.